Difference between revisions of "Turbidity sensors"
(→Turbidity Sensors) |
|||
| Line 9: | Line 9: | ||
The first attempt to measure turbidity in a standardized fashion was made by Whipple and Jackson in 1900. Whipple and Jackson developed a standard suspension fluid using 1,000 parts per million (ppm) of diatomaceous earth in distilled water to use as a scale and a turbidimeter (Jackson candle turbidimeter). The method consists in placing a lit candle under a flat-bottomed tube in which the water in poured until the image of the candles fades unto a glare when observed from above. Visual image extinction occurred when the intensity of the scattered light equaled that of transmitted light. The height of the water would be read against the ppm-silica scale and units were given in Jackson turbidity units (JTU). Needless to say that this method is prone to errors too, as the diatomaceous earth varies in its composition and the reading is subjected to the observer. | The first attempt to measure turbidity in a standardized fashion was made by Whipple and Jackson in 1900. Whipple and Jackson developed a standard suspension fluid using 1,000 parts per million (ppm) of diatomaceous earth in distilled water to use as a scale and a turbidimeter (Jackson candle turbidimeter). The method consists in placing a lit candle under a flat-bottomed tube in which the water in poured until the image of the candles fades unto a glare when observed from above. Visual image extinction occurred when the intensity of the scattered light equaled that of transmitted light. The height of the water would be read against the ppm-silica scale and units were given in Jackson turbidity units (JTU). Needless to say that this method is prone to errors too, as the diatomaceous earth varies in its composition and the reading is subjected to the observer. | ||
| − | + | {|style="margin: 0.5em 0.5em 0.5em 0.5em;border: 1px solid #00787A;" cellspacing="1" cellpadding="5" width="750" align="right" | |
| − | ==== | + | | |
| + | <div style="background-color:#00787A; padding:0.5em 1em 0.5em 1em; font-size:120%; font-weight:bold; "><span style="color:#FFFFFF">Light Scattering different from Particle Concentration</span></div> | ||
| + | {|border="0" cellspacing="0" cellpadding="0" width="100%" | ||
| + | |-valign="top" | ||
| + | |<div style="text-align: justify; font-size:95%;padding:1em 1em 1em 1em;"><span style="color:#00787A"> | ||
| + | |||
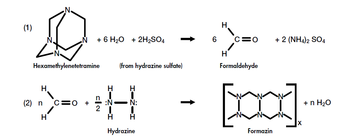
| + | '''Formazin''' | ||
In 1926, Kingsbury and Clark discovered formazin, a suspension that is created by the polymerization of hexamethylenetetramine and hydrazine sulfate in water. This new suspension improved greatly the consistency in standards formulation. Measuring units were renamed to formazin turbidity units (FTU). Several techniques were developed throughout the years with different light sources and scale systems, but in the end they were all still dependent on the observer's subjectivity. | In 1926, Kingsbury and Clark discovered formazin, a suspension that is created by the polymerization of hexamethylenetetramine and hydrazine sulfate in water. This new suspension improved greatly the consistency in standards formulation. Measuring units were renamed to formazin turbidity units (FTU). Several techniques were developed throughout the years with different light sources and scale systems, but in the end they were all still dependent on the observer's subjectivity. | ||
| + | |||
| + | {| style="float: right;" border="0" | ||
| + | |[[Image: formazin.PNG| thumb|350px| Synthesis of formazin.]] | ||
| + | |} | ||
| + | |||
In addition to the problems mentioned above, the wavelength of the candle light is not as effectively back-scattered by small size particles while the dark particles absorb so much more light than they scatter, that it is impossible to obtain a reading. | In addition to the problems mentioned above, the wavelength of the candle light is not as effectively back-scattered by small size particles while the dark particles absorb so much more light than they scatter, that it is impossible to obtain a reading. | ||
Revision as of 09:19, 30 July 2012
Contents
[hide]Introduction
Definition
Turbidity is defined as the reduction of transparency of a liquid caused by the presence of undissolved suspended matter.[1] The origin of the particles found in seawater can be mineral (such as clay and silts) or organic (such as particulate organic matter or living organisms like plankton). Turbidity is not, however, a direct measure of suspended particles in water, but a measure of the scattering effect such particles have on light.
Measurements and Standards of Turbidity throughout history
Secchi Disk
The first record of turbidity being approached in a scientific way, is attributed to the head of the Papal Navy in 1865, Commander Cialdi. Commander Cialdi was interested in the transparency of the sea and the visibility of its floor (for navigational purposes) and during his research he had read that a captain had reported seeing a plate in a net at a depth of 40 m. Commander Cialdi commenced his investigation on the sea visibility and immediately tried several different disc sizes and colors. Later, he hired the services of Professor Pietro Angelo Secchi, an Italian scholar and priest. Together they published Sur la Transparence de la Mer.(On the transparency of the Sea) where they described the development of a white disk connected to a pole or a cable to measure the transparency of the sea. Secchi made further observations on the influence of the boat's shadow, the surface reflection of the light, the clearness of the sky, and the height at which the observer stands[2]. While his method has been used ever since and it is still used today as a qualitative measure of the oceans' turbidity, it has never been standardized so its accuracy is very limited.
Jackson candle turbidimeter
The first attempt to measure turbidity in a standardized fashion was made by Whipple and Jackson in 1900. Whipple and Jackson developed a standard suspension fluid using 1,000 parts per million (ppm) of diatomaceous earth in distilled water to use as a scale and a turbidimeter (Jackson candle turbidimeter). The method consists in placing a lit candle under a flat-bottomed tube in which the water in poured until the image of the candles fades unto a glare when observed from above. Visual image extinction occurred when the intensity of the scattered light equaled that of transmitted light. The height of the water would be read against the ppm-silica scale and units were given in Jackson turbidity units (JTU). Needless to say that this method is prone to errors too, as the diatomaceous earth varies in its composition and the reading is subjected to the observer.
|
Light Scattering different from Particle Concentration
|