Difference between revisions of "Methylmercury"
| Line 10: | Line 10: | ||
| align="center" bgcolor="#FFFFFF" | [[Image:methylmercury.png|200px|methylmercury]] | | align="center" bgcolor="#FFFFFF" | [[Image:methylmercury.png|200px|methylmercury]] | ||
|- | |- | ||
| − | | align="center" | X is a random ion | + | | align="center" | X<sup>-</sup> is a random ion |
|- | |- | ||
|} | |} | ||
Revision as of 14:48, 24 July 2009
Notes
| methylmercury |
|---|
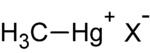
|
| X- is a random ion |
While inorganic mercury is the dominant form, most mercury which accumulates in benthic invertabrates and fish is methylmercury. Unlike most other metals, methylmercury biomagnifies through the food chain. As such methylmercury is expected to be most hazardous for organisms on the top of the food chain. So contain high trophic level carnivorous fish (like mackerel and tuna) to contain 10.000 times more methylmercury than the surrounding environment. Species eating these fish (humans,Sea birds and marine mammals) are expected to have even more problems. However while this holds true for humans, marine birds and marine mammals appear to be quite resistant towards methylmercury accumulation.
Humans acquire methylmercury through consumption of fish and mussels. Usually this doesn't pose any health risks, although high consumption of predatory fish (with high mercury content) by pregnant women, might pose problems for the young and unborn babies. You can also verify whether the fish you eat has been caught in a sustainable manner.[2]
See also
PCB and heavy metals in beached sperm whales
References
- ↑ Lawrence E (ed.), 2000. Henderson’s Dictionary of Biological Terms. 12th edition. Prentice Hall, Pearson Education Limited. Harlow, Great Britain.
- ↑ http://www.lenntech.com/Periodic-chart-elements/Hg-en.htm