Difference between revisions of "Trichlorobenzene"
| Line 32: | Line 32: | ||
TCBs are not biodegradable, very toxic to aquatic organisms and may cause long term adverse effects in the aquatic environment. They have a high tenancy towards [[bioaccumulation]]. | TCBs are not biodegradable, very toxic to aquatic organisms and may cause long term adverse effects in the aquatic environment. They have a high tenancy towards [[bioaccumulation]]. | ||
| + | |||
| + | |||
| + | == Environmental standards and legislation == | ||
| + | |||
| + | [[OSPAR List of priority substances|Included in the OSPAR list of substances of priority action]] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | == See also == | ||
| + | |||
| + | [http://www.vliz.be/projects/endis/EDnorth.php?showchemprop=true&showeffects=true&chemeffects=true&chemid=656 Tetrabromobisphenol-A on ED North Database] | ||
| + | <P> | ||
| + | [http://www.ospar.org/documents%5Cdbase%5Cpublications%5Cp00202_BD%20on%20TBBPA.pdf OSPAR background document on cadmium] | ||
| + | <P> | ||
| + | <BR> | ||
| + | <P> | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | |||
| + | [[Category:Coastal and marine pollution]] | ||
Revision as of 11:44, 3 August 2009
Definition of trichlorobenzene:
Trichlorobenzenes are cyclic aromatic compounds formed by the addition of 3 atoms of chlorine to the benzene ring. There are 3 isomers: 1,2,3-trichlorobenzene (1,2,3-TCB), 1,2,4-trichlorobenzene (1,2,4-TCB) and 1,3,5-trichlorobenzene (1,3,5-TCB).
[1]
This is the common definition for trichlorobenzene, other definitions can be discussed in the article
|
Notes
| Trichlorobenzene |
|---|
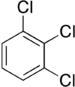
|
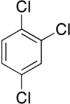
|
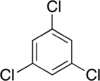
|
| Formula |
| C6H3Cl3 |
The EU production of TCBs in 2003 was estimated below 4 000 tonnes, of which a high amount is exported. Today TCBs are be used as intermediate in the production of herbicides and pesticides. However they were historically used as dye carriers, which adsorb into the polyester fibers. During dying a significant amount of TCBs were emitted to waste waters. It has also been used as an additive to PCBs for insulating and cooling dielectric fluids.
When entered into the marine environment, TCBs will most likely be evaporated, or adsorb to organic sediments. It's also thought that during transport to the sea, TCBs are adsorbed to the riverine sediments. This results in high concentrations in river sediments, making them "pollution hot spots". They are immobile and very persistent in these soils.
TCB are not considered to be carcinogenic. It has been show to cause acute toxicity in algae, crustaceans and fish at a concentration of 1,4 mg/l, 0,45 mg/l and 21 mg/l respectively. Concentrations in marine waters range from 0,002 µg/l (in open ocean) to 0,03 µg/l (in polluted areas). The highest environment concentrations measured in marine fish range from 0,14 to 2,3 µg/kg lipid weight. TCBs are also thought to have reproductive and endocrine disrupting effects.
TCBs are not biodegradable, very toxic to aquatic organisms and may cause long term adverse effects in the aquatic environment. They have a high tenancy towards bioaccumulation.
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
See also
Tetrabromobisphenol-A on ED North Database
OSPAR background document on cadmium