Salinity sensors

|

|
The authors below are planning to work on this article
See also: Instruments and sensors to measure environmental parameters
Contents
Salinity Definition
Salinity is the amount ( in grams) of dissolved solid material in a kilogram of seawater after all the bromine has been replaced by an equivalent quantity of chlorine, all the carbonate converted to oxide, and all of the organic matter destroyed. [1] [2] This definition is impractical and the procedure difficult to carry out with precision. Alternatively, other parameters have been used in recent history as a proxy to calculate salinity.
Salinity measurements and definitions throughout history
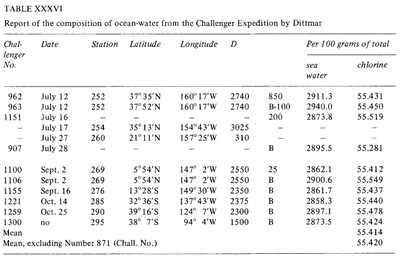
Although attempts have been made throughout history (as far as Ancient Greece times) to address the "saltiness" of seawater, the low sensitivity of the analytic methods meant that measurements were not sufficiently accurate to be considered. During the Modern History new and more precise methodologies were developed: weighing after evaporation (Boyle,1693; see Birch, 1965), solvent extraction (Lavoisier, 1772) and precipitation (Bergman, 1784). In 1865 Forchhammer introduced the term salinity and dedicated himself to measure individual components of seasalt rather than the total salinity. He found that the ratio of major salts in samples of seawater from various locations was constant. This constant ratio is known as Forchhammer's Principle, or the Principle of Constant Proportions, from which the salinity could be calculated from the chlorine content. Towards the end of the nineteenth century, William Dittmar, following the work of Forchhammer, tested several methods to analyse the salinity and chemical composition of seawater. The Dittmar methods for chemical analysis of the seawater were extremely precise. Dittmar analysed the chlorine content in seawater using silver nitrate precipitation of the chloride, and compared it with synthetically prepared seawater samples to vouch for the method's accuracy. Dittmar later analysed 77 samples taken during the Challenger expedition and noticed the same constancy of composition observed by Forchhammer: "although the concentration of the waters is very different, the percentage composition of the dissolved material is almost the same is all cases". [3]
Sensors
Salinity is a ratio and not a physical parameter that can be measured. Thus, “Salinity sensors” do not exist. There are several different ways to calculate this ratio with pros and cons for each method.
Salinity Scales
Refractometer
Conductivity meter
Calibration
Literature
... ...
External Links
... ...
References
- ↑ http://www.aslo.org/lo/toc/vol_14/issue_3/0437.pdf
- ↑ KNUDSEN, M. 1901. Hydrographical tables. G.E.C. Gad, Copenhagen, 63p
- ↑ William J. Wallace (1974). The Development of the Chlorinity/Salinity Concept in Oceanography. Amsterdam: Elsevier. 239.