Difference between revisions of "Testpage3"
Dronkers J (talk | contribs) |
Dronkers J (talk | contribs) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Review | |
| + | |name=Job Dronkers|AuthorID=120| | ||
| + | }} | ||
| − | + | '''Resilience and resistance''' | |
| − | |||
| + | {{Definition|title=Resistance | ||
| + | |definition= The capacity to weather a disturbance without loss (Lake 2013<ref name=L>Lake, P.S. 2013. Resistance, Resilience and Restoration. Ecological Management and Restoration 14: 20-24</ref>). }} | ||
| − | |||
| − | + | {{Definition|title=Resilience | |
| + | |definition=(1) the capability to anticipate, prepare for, respond to, and recover from significant multihazard threats with minimum damage to social well-being, the economy, and the environment (sometimes called 'socio-ecological resilience')(Olsen et al. 2019<ref name=O>Olsson, S., Melvin, A. and Giles, S. (eds.) 2019. Climate change and ecosystems. Procs. Sackler Forum on Climate Change and Ecosystems, Washington, DC, November 8-9, 2018, organized by the National Academy of Sciences and The Royal Society</ref>); | ||
| + | (2) the capability of a (socio-)ecological system to remain within a stability domain when subjected to environmental change, while continually changing and adapting yet remaining within critical thresholds (sometimes called 'general resilience') (Folke et al. 2010<ref name=F>Folke, C., Carpenter, S. R., Walker, B., Scheffer, M., Chapin, T. and Rockstrom, J. 2010. Resilience thinking: integrating resilience, adaptability and transformability. Ecology and Society 15(4): 20</ref>; Scheffer 2009<ref>Scheffer, M. 2009. Critical transitions in nature and society. Princeton University Press, Princeton, New Jersey, USA</ref>; Brand and Jax 2007<ref name=BJ>Brand, F.S. and K. Jax. 2007. Focusing the meaning(s) of resilience: resilience as a descriptive concept and a boundary object. Ecology and Society 12(1):23</ref>); | ||
| − | + | (3) the capacity to experience shocks while retaining essentially the same function, structure, feedbacks, and therefore identity (sometimes called 'ecological resilience') (Brand and Jax 2007<ref name=BJ/>; DEFRA 2019<ref name=DEFRA>Haines‐Young, R. and Potschin. M. (eds.) 2010. The Resilience of Ecosystems to Environmental Change (RECCE). Overview Report, 27 pp. Defra Project Code: NR0134</ref>), which is closely related to the concept of 'ecosystem resistance': the amount of disturbance that a system can withstand before it shifts into a new regime or an alternative stable state (Holling 1973<ref>Holling, C.S. 1973. Resilience and stability of ecological systems. Annual Rev. Ecol. Syst. 4: 1–23. doi: 10.1146/annurev.es.04.110173.000245</ref>; Gunderson 2000<ref>Gunderson, L.H. 2000. Ecological Resilience - in Theory and Application. Annual Review of Ecology and Systematics 31:425-439.</ref>); | |
| + | (4) the capacity of an ecosystem to regain its fundamental structure, processes, and functioning (or remain largely unchanged) despite stresses, disturbances, or invasive species (e.g., Hirota et al., 2011<ref>Hirota,M., Holmgren,M., Van Nes, E. H, and Scheffer,M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334: 232–235. doi: 10.1126/science.1210657</ref>; Chambers et al., 2014<ref>Chambers, J. C., Bradley, B. A., Brown, C. S., D’Antonio, C., Germino, M. J., Grace, J. B., et al. 2014. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in the cold desert shrublands of western North America. Ecosystems 7: 360–375. doi: 10.1007/s10021-013-9725-5</ref>; Pope et al., 2014<ref>Pope, K. L., Allen, C. R., and Angeler, D. G. 2014. Fishing for resilience. T. N. Am. Fisheries Soc. 143: 467–478. doi: 10.1080/00028487.2014.880735</ref>; Seidl et al., 2016<ref>Seidl, R., Spies, T. A., Peterson, D. L., Stephens, S. L., and Hick, J. A. 2016. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J. Appl. Ecol. 53 : 120–129. doi: 10.1111/1365-2664.12511</ref>), which can be measured by the time needed to recover its original state (sometimes called 'engineering resilience'<ref name=L>Lake, P.S. 2013. Resistance, Resilience and Restoration. Ecological Management and Restoration 14: 20-24</ref>). | ||
| + | }} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ==Introduction== | |
| + | Coastal and marine ecosystems are affected by environmental disturbance at a variety of spatio-temporal scales. The organisms inhabiting these systems are adapted to such disturbance, either by being tolerant of these conditions or by playing a role in one or more of the successional stages that follow during ecosystem recovery. | ||
| − | + | If all species in the system were tolerant to a particular perturbation, very little would change at the ecosystem level, and we could call the system resistant to this disturbance. However, often a disturbance, such as a temporary very low oxygen level, affects a substantial proportion of the organisms dramatically, either causing them to die, or forcing them to rapidly migrate to more favorable parts of the environment. Such an adverse disturbance could locally defaunate a certain volume in the pelagic or a certain area of hard or soft substrate. Such destruction at a local scale does not mean the end of local functioning. Usually organisms are available at a larger spatial scale that can re-colonize the affected area, according to their particular tolerances and abilities to favorably affect their local environment. | |
| − | + | The term resilience has been defined in different ways, illustrated in the definition above. According to DEFRA (2019<ref name=DEFRA/>) there is limited consensus in the literature about how resilience can be characterized and assessed. The term resilience is sometimes used to represent some kind of normative proposition about what kinds of ecosystem characteristics are desirable or necessary in the context of sustainable development, reflecting particular cultural and philosophical assumptions<ref name=DEFRA/>. However, the resistance of an ecosystem (see the definition above) to changing conditions and the rate of recovery following some disruptive event are generally considered major components of resilience that can in principle be expressed in quantitative terms. | |
| + | Other attributes such as the capacity of ecosystems to transform and adapt in the face of environmental change (i.e. system's ability to re-organize itself) are more difficult to translate to practice. According to Dawson et al. (2010<ref name=D>Dawson, T.P., Rounsevell, M.D.A., Kluvankova‐Oravska, T., Chobotova V. and Stirling, A. 2010. Dynamic properties of complex adaptive ecosystems: implications for the sustainability of services provision. Biodiversity and Conservation 19: 2843‐2853</ref>), resilience concerns the response of ecosystems to changing environmental conditions and must be looked at alongside other additional dynamic features, namely durability, robustness and stability. These concepts can be defined as<ref name=D/>: | ||
| + | * Durability: ability to cope with a chronic stress, but the source of this stress is endogenous; | ||
| + | * Robustness: ability to recover or maintain the systems' social-ecological functions in the face of an external and chronic driver; | ||
| + | * Stability: system’s tolerance to transient and endogenous shocks or disruptions. | ||
| − | + | Both resistance and resilience cause an ecosystem to remain relatively unchanged when confronted to a disturbance, but in the case of resistance no internal re-organization and successional change is involved. In contrast, resilience implies that the system is internally re-organizing, perhaps through a mozaic of patches that are at different stages of re-assembly. System responses to changing environmental conditions are displayed schematically in Fig. 1, corresponding to different resilience characteristics. | |
| − | |||
| − | [[Image: | + | [[Image:ResilienceTrajectories.jpg|thumb|900px|center|Figure 1. Schematic representation of the trajectories of a (socio-)ecological system in a plane defined by the system state (fundamental structure, processes, and functioning - vertical axis) and the change of environmental conditions (horizontal axis), for different resilience characteristics (a, b, c, d). The initial state corresponds to the position on the graph at the vertical axis (zero change in environmental conditions). In all situations the ecosystem is assumed to collapse irreversibly (down to the horizontal axis) when the change in environmental conditions is much greater than the systems' resistance. The angle <math>\alpha</math> represents the rate at which the system recovers when the change in environmental conditions is reduced (small <math>\alpha</math> means slow recovery, large <math>\alpha</math> means fast recovery). Panel a: Resilience characterized by high resistance (definition 3) and slow recovery (definition 4). Panel b: Resilience characterized by low resistance and fast recovery. Panel c: Resilience characterized by a shift to an alternative stable system state. Panel d: Low resilience, characterized by low resistance and slow recovery.]] |
| + | When considering the potential effect of a certain type of disturbance it is thus useful to ask two questions: | ||
| + | # Will the species of this system be able to tolerate it (implying resistance), and if not, | ||
| + | # Is recovery possible through a successional trajectory, back to the same, or at least a desirable, ecosystem state (implying resilience)? | ||
| + | Resistance breaks down when uni-directional ongoing change acts faster than the organisms' ability to adapt their tolerances. If uni-directional ongoing change is this fast (even if gradual), the system will not be sufficiently resilient either, as full recovery through succession will then not be possible. Recovery from sudden and local disturbance is often possible through recolonization, but the rate of recovery will depend crucially on the spatial extent of disturbance. For example, recovery from anoxia could take 5 to 8 months at the scale of square meters (Rossi et al. 2009<ref name=R>Rossi, F., Vos, M. & Middelburg, J.J. 2009. Species identity, diversity and microbial carbon flow in reassembling macrobenthic communities. Oikos 118: 503-512.</ref>), but could take 5 to 8 years at the scale of a whole bay (Diaz & Rosenberg 1995<ref>Diaz, R.J. & Rosenberg, R. 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33:245-303.</ref>). | ||
| + | According to definition (4), the speed at which an ecosystem returns to its former state following a (minor) disturbance can be considered a measure of resilience. The idea is that a system with a short return time is more resilient than one with a long return time. Such resilience measured as (1 / the return time to a stable equilibrium) has also been called ''engineering resilience''. It has however a long history of use among ecologists (Pimm 1982<ref>Pimm, S.L. 1982. Food Webs. The University of Chicago Press.</ref>, DeAngelis 1992<ref>DeAngelis, D.L. 1992. Dynamics of Nutrient Cycling and Food Webs. Chapman and Hall, London.</ref>, Vos et al. 2005<ref>Vos, M., Kooi, B.W., DeAngelis, D.L. & Mooij, W.M. 2005. Inducible defenses in food webs. In: Dynamic Food Webs. Multispecies Assemblages, Ecosystem Development and Environmental Change. Eds. P.C. de Ruiter, V. Wolters & J.C. Moore. Academic Press. Pp. 114-127.</ref>). Resilience is also used in a way that more closely resembles the definition of resistance. ''Ecological resilience'' was defined as the amount of disturbance that an ecosystem could withstand without changing self-organized processes and structures (definition 3). | ||
| − | + | Resilience of coastal systems largely depends on biodiversity, which is a major requirement for allowing ecosystems to adapt to changing conditions. The human impact on the environment through pollution, fisheries, sediment erosion / deposition and global climate change has brought about much faster change than would occur under natural conditions, putting severe stress on many ecosystems. Without genetic diversity, natural selection cannot occur and if natural selection is limited, adaptation is impossible. Preservation of biodiversity and, more specifically, genetic diversity is therefore of paramount importance for successful adaptation to our rapidly changing environments. However, biodiversity may not always protect ecosystems from major abiotic disturbances (Folke et al. 2004<ref>Folke, C., Carpenter, S., Walker, B., Scheffer, M., Elmqvist, T., Gunderson, L. & Holling, C.S. 2004. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annual Review of Ecolog and Systematics 35:557-581.</ref>). | |
| + | ==Resilience through recolonization== | ||
| + | To understand resilience of ecosystems it is essential to understand what drives succession within these ecosystems. Succession determines how, and how fast, communities return to their original state, or perhaps enter a new state. Many aspects of succession can be understood in terms of trade-offs between the ability to be either a good early (re)colonizer, or a good competitor. Succession involves a gradual replacement of colonizer/competitor species according to the degree to which they tolerate, facilitate or inhibit certain environmental conditions and other species (Rossi et al. 2009<ref name=R/>). The extent to which processes of (re)colonization and succession can take place largely determines the recovery of ecosystems after major disruption and is therefore an essential characteristic of the resilience of ecosystems. | ||
| + | In this context, it is important to consider the spatial component of ecosystem resilience. Diversity of structurally and functionally connected landscapes, rich in resources and species, promotes the flow or movement of individuals, genes, and ecological processes. Below certain thresholds of connectivity the capacity to regain structure and function after perturbation is lost (Holl and Aide, 2011; Rudnick et al., 2012;McIntyre et al., 2014; Rappaport et al., 2015; Ricca et al., 2018). Chambers et al. (2019<ref name=CAC>Chambers, J.C., Allen, C.R. and Cushman, S.A. 2019. Operationalizing Ecological Resilience Concepts for Managing Species and Ecosystems at Risk. Front. Ecol. Evol. 7:241. doi: 10.3389/fevo.2019.00241</ref>), based on Allen et al. (2016<ref> Allen, C. R., Angeler, D. G., Cumming, G. S., Folk, C., Twidwell, D., and Uden, D. R. 2016. Quantifying spatial resilience. J. Appl. Ecol. 53, 625–635. doi: 10.1111/1365-2664.12634</ref>), have therefore introduced the concept of 'spatial resilience', which is a measure of how spatial attributes, processes, and feedbacks vary over space and time in response to disturbances and affect the resilience of ecosystems. Self-organization through strong feedbacks at multiple scales and high levels of functional diversity and redundancy, stabilizes the system with respect to disturbances within the range of historic variability. | ||
| + | When creating Marine Protected Areas, the sources of populations at all stages of succession should be protected, to preserve 'ecological memory' to the fullest possible extent. This includes protecting not only 'high quality' habitats that harbour healthy mature communities, but also 'low quality' and disturbed habitats that are required for those species that contribute to early recovery of perturbed areas (Rossi et al. 2009<ref name=R/>). The selection of Marine Protected Areas thus involves evaluating | ||
| + | the number, size, and spatial configuration of habitat fragments and degree of connectivity required to support restoration of ecosystems and conservation of focal habitats and species<ref name=CAC/><ref name=O/>. | ||
| + | ==Resistance to changes in abiotic and biotic factors== | ||
| − | + | Community composition and ecosystem function may change very little under environmental change when the organisms can adapt to such change or tolerate it for some time (when the change is only temporary). However, all organisms have bounds to what they can temporarily or permanently tolerate, and when change exceeds some of these limits, the community composition and ecosystem functioning is likely to change. | |
| − | + | It is unlikely that communities can be resistant to ongoing gradual change, such as global warming. Acclimation and phenotypic plasticity do not suffice to maintain the system as it is. Genetic adaptation could allow community members to track such abiotic environmental change, but it is more likely that the area where the community is functioning will be invaded by species that function well at higher temperatures. The original species will thus have to deal with new competitors and predators, in addition to the changed abiotic factor. To some extent the original community can track the preferred temperature range, by moving spatially to greater depths or to alternative geographic areas. But these new areas are likely to differ in other ecological aspects such as water pressure, light climate and perhaps speeds of water flow etc. | |
| + | ==Adaptation and the consequences of mortality at different trophic levels== | ||
| + | External disturbance interacts with internal mechanisms that shape community structure. To understand how an increased mortality of top-predators will affect the entire food chain, it is essential to understand how processes of mutual adaptation within food chains already give shape to existing patterns such as trophic structure (how biomass in ecosystems is partitioned between trophic levels). | ||
| + | Abundances at different trophic levels (such as algae, herbivores, carnivores and top-predators) and their responses to increased mortality (as under environmental change) depend critically on different mechanisms of adaptation within food chains and on the importance of population density at each of these trophic levels. However, different types of adaptation to living in a food chain context (balancing the need to acquire resources with the need to avoid predation) can often have similar consequences. For example, micro-evolution of behaviour, species replacement and induced defenses at a middle trophic level may all have similar effects on trophic level abundances in disturbed food chains (Abrams and Vos 2003<ref>Abrams, P.A & Vos, M. 2003. Adaptation, density dependence and the responses of trophic level abundances to mortality. Evolutionary Ecology Research 5: 1113-1132</ref>). | ||
| − | + | ==Related articles== | |
| − | + | :[[Integrated Coastal Zone Management (ICZM)]] | |
| − | + | :[[Thresholds of environmental sustainablility]] | |
| − | + | :[[Sustainability indicators]] | |
| − | |||
| − | |||
| − | == | ||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | ==References== | ||
| + | <references/> | ||
| − | |||
| − | + | <br> | |
| − | |||
| + | {{author | ||
| + | |AuthorID=11928 | ||
| + | |AuthorFullName=Vos, Matthijs | ||
| + | |AuthorName=Matthijs}} | ||
| − | + | [[Category:Coastal and marine ecosystems]] | |
| − | + | [[Category:Integrated coastal zone management]] | |
| − | |||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 15:04, 24 August 2020
Resilience and resistance
Definition of Resistance:
The capacity to weather a disturbance without loss (Lake 2013[1]).
This is the common definition for Resistance, other definitions can be discussed in the article
|
Definition of Resilience:
(1) the capability to anticipate, prepare for, respond to, and recover from significant multihazard threats with minimum damage to social well-being, the economy, and the environment (sometimes called 'socio-ecological resilience')(Olsen et al. 2019[2]);
(2) the capability of a (socio-)ecological system to remain within a stability domain when subjected to environmental change, while continually changing and adapting yet remaining within critical thresholds (sometimes called 'general resilience') (Folke et al. 2010[3]; Scheffer 2009[4]; Brand and Jax 2007[5]); (3) the capacity to experience shocks while retaining essentially the same function, structure, feedbacks, and therefore identity (sometimes called 'ecological resilience') (Brand and Jax 2007[5]; DEFRA 2019[6]), which is closely related to the concept of 'ecosystem resistance': the amount of disturbance that a system can withstand before it shifts into a new regime or an alternative stable state (Holling 1973[7]; Gunderson 2000[8]); (4) the capacity of an ecosystem to regain its fundamental structure, processes, and functioning (or remain largely unchanged) despite stresses, disturbances, or invasive species (e.g., Hirota et al., 2011[9]; Chambers et al., 2014[10]; Pope et al., 2014[11]; Seidl et al., 2016[12]), which can be measured by the time needed to recover its original state (sometimes called 'engineering resilience'[1]).This is the common definition for Resilience, other definitions can be discussed in the article
|
Contents
Introduction
Coastal and marine ecosystems are affected by environmental disturbance at a variety of spatio-temporal scales. The organisms inhabiting these systems are adapted to such disturbance, either by being tolerant of these conditions or by playing a role in one or more of the successional stages that follow during ecosystem recovery.
If all species in the system were tolerant to a particular perturbation, very little would change at the ecosystem level, and we could call the system resistant to this disturbance. However, often a disturbance, such as a temporary very low oxygen level, affects a substantial proportion of the organisms dramatically, either causing them to die, or forcing them to rapidly migrate to more favorable parts of the environment. Such an adverse disturbance could locally defaunate a certain volume in the pelagic or a certain area of hard or soft substrate. Such destruction at a local scale does not mean the end of local functioning. Usually organisms are available at a larger spatial scale that can re-colonize the affected area, according to their particular tolerances and abilities to favorably affect their local environment.
The term resilience has been defined in different ways, illustrated in the definition above. According to DEFRA (2019[6]) there is limited consensus in the literature about how resilience can be characterized and assessed. The term resilience is sometimes used to represent some kind of normative proposition about what kinds of ecosystem characteristics are desirable or necessary in the context of sustainable development, reflecting particular cultural and philosophical assumptions[6]. However, the resistance of an ecosystem (see the definition above) to changing conditions and the rate of recovery following some disruptive event are generally considered major components of resilience that can in principle be expressed in quantitative terms.
Other attributes such as the capacity of ecosystems to transform and adapt in the face of environmental change (i.e. system's ability to re-organize itself) are more difficult to translate to practice. According to Dawson et al. (2010[13]), resilience concerns the response of ecosystems to changing environmental conditions and must be looked at alongside other additional dynamic features, namely durability, robustness and stability. These concepts can be defined as[13]:
- Durability: ability to cope with a chronic stress, but the source of this stress is endogenous;
- Robustness: ability to recover or maintain the systems' social-ecological functions in the face of an external and chronic driver;
- Stability: system’s tolerance to transient and endogenous shocks or disruptions.
Both resistance and resilience cause an ecosystem to remain relatively unchanged when confronted to a disturbance, but in the case of resistance no internal re-organization and successional change is involved. In contrast, resilience implies that the system is internally re-organizing, perhaps through a mozaic of patches that are at different stages of re-assembly. System responses to changing environmental conditions are displayed schematically in Fig. 1, corresponding to different resilience characteristics.
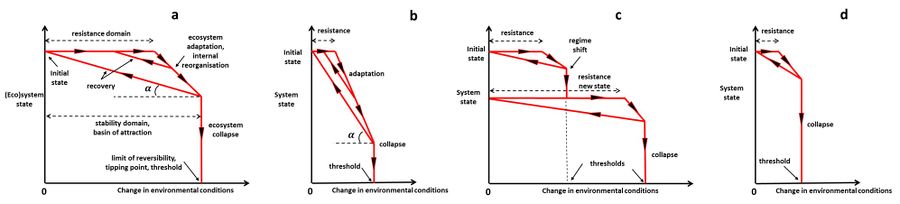
When considering the potential effect of a certain type of disturbance it is thus useful to ask two questions:
- Will the species of this system be able to tolerate it (implying resistance), and if not,
- Is recovery possible through a successional trajectory, back to the same, or at least a desirable, ecosystem state (implying resilience)?
Resistance breaks down when uni-directional ongoing change acts faster than the organisms' ability to adapt their tolerances. If uni-directional ongoing change is this fast (even if gradual), the system will not be sufficiently resilient either, as full recovery through succession will then not be possible. Recovery from sudden and local disturbance is often possible through recolonization, but the rate of recovery will depend crucially on the spatial extent of disturbance. For example, recovery from anoxia could take 5 to 8 months at the scale of square meters (Rossi et al. 2009[14]), but could take 5 to 8 years at the scale of a whole bay (Diaz & Rosenberg 1995[15]).
According to definition (4), the speed at which an ecosystem returns to its former state following a (minor) disturbance can be considered a measure of resilience. The idea is that a system with a short return time is more resilient than one with a long return time. Such resilience measured as (1 / the return time to a stable equilibrium) has also been called engineering resilience. It has however a long history of use among ecologists (Pimm 1982[16], DeAngelis 1992[17], Vos et al. 2005[18]). Resilience is also used in a way that more closely resembles the definition of resistance. Ecological resilience was defined as the amount of disturbance that an ecosystem could withstand without changing self-organized processes and structures (definition 3).
Resilience of coastal systems largely depends on biodiversity, which is a major requirement for allowing ecosystems to adapt to changing conditions. The human impact on the environment through pollution, fisheries, sediment erosion / deposition and global climate change has brought about much faster change than would occur under natural conditions, putting severe stress on many ecosystems. Without genetic diversity, natural selection cannot occur and if natural selection is limited, adaptation is impossible. Preservation of biodiversity and, more specifically, genetic diversity is therefore of paramount importance for successful adaptation to our rapidly changing environments. However, biodiversity may not always protect ecosystems from major abiotic disturbances (Folke et al. 2004[19]).
Resilience through recolonization
To understand resilience of ecosystems it is essential to understand what drives succession within these ecosystems. Succession determines how, and how fast, communities return to their original state, or perhaps enter a new state. Many aspects of succession can be understood in terms of trade-offs between the ability to be either a good early (re)colonizer, or a good competitor. Succession involves a gradual replacement of colonizer/competitor species according to the degree to which they tolerate, facilitate or inhibit certain environmental conditions and other species (Rossi et al. 2009[14]). The extent to which processes of (re)colonization and succession can take place largely determines the recovery of ecosystems after major disruption and is therefore an essential characteristic of the resilience of ecosystems.
In this context, it is important to consider the spatial component of ecosystem resilience. Diversity of structurally and functionally connected landscapes, rich in resources and species, promotes the flow or movement of individuals, genes, and ecological processes. Below certain thresholds of connectivity the capacity to regain structure and function after perturbation is lost (Holl and Aide, 2011; Rudnick et al., 2012;McIntyre et al., 2014; Rappaport et al., 2015; Ricca et al., 2018). Chambers et al. (2019[20]), based on Allen et al. (2016[21]), have therefore introduced the concept of 'spatial resilience', which is a measure of how spatial attributes, processes, and feedbacks vary over space and time in response to disturbances and affect the resilience of ecosystems. Self-organization through strong feedbacks at multiple scales and high levels of functional diversity and redundancy, stabilizes the system with respect to disturbances within the range of historic variability.
When creating Marine Protected Areas, the sources of populations at all stages of succession should be protected, to preserve 'ecological memory' to the fullest possible extent. This includes protecting not only 'high quality' habitats that harbour healthy mature communities, but also 'low quality' and disturbed habitats that are required for those species that contribute to early recovery of perturbed areas (Rossi et al. 2009[14]). The selection of Marine Protected Areas thus involves evaluating the number, size, and spatial configuration of habitat fragments and degree of connectivity required to support restoration of ecosystems and conservation of focal habitats and species[20][2].
Resistance to changes in abiotic and biotic factors
Community composition and ecosystem function may change very little under environmental change when the organisms can adapt to such change or tolerate it for some time (when the change is only temporary). However, all organisms have bounds to what they can temporarily or permanently tolerate, and when change exceeds some of these limits, the community composition and ecosystem functioning is likely to change.
It is unlikely that communities can be resistant to ongoing gradual change, such as global warming. Acclimation and phenotypic plasticity do not suffice to maintain the system as it is. Genetic adaptation could allow community members to track such abiotic environmental change, but it is more likely that the area where the community is functioning will be invaded by species that function well at higher temperatures. The original species will thus have to deal with new competitors and predators, in addition to the changed abiotic factor. To some extent the original community can track the preferred temperature range, by moving spatially to greater depths or to alternative geographic areas. But these new areas are likely to differ in other ecological aspects such as water pressure, light climate and perhaps speeds of water flow etc.
Adaptation and the consequences of mortality at different trophic levels
External disturbance interacts with internal mechanisms that shape community structure. To understand how an increased mortality of top-predators will affect the entire food chain, it is essential to understand how processes of mutual adaptation within food chains already give shape to existing patterns such as trophic structure (how biomass in ecosystems is partitioned between trophic levels).
Abundances at different trophic levels (such as algae, herbivores, carnivores and top-predators) and their responses to increased mortality (as under environmental change) depend critically on different mechanisms of adaptation within food chains and on the importance of population density at each of these trophic levels. However, different types of adaptation to living in a food chain context (balancing the need to acquire resources with the need to avoid predation) can often have similar consequences. For example, micro-evolution of behaviour, species replacement and induced defenses at a middle trophic level may all have similar effects on trophic level abundances in disturbed food chains (Abrams and Vos 2003[22]).
Related articles
- Integrated Coastal Zone Management (ICZM)
- Thresholds of environmental sustainablility
- Sustainability indicators
References
- ↑ 1.0 1.1 Lake, P.S. 2013. Resistance, Resilience and Restoration. Ecological Management and Restoration 14: 20-24
- ↑ 2.0 2.1 Olsson, S., Melvin, A. and Giles, S. (eds.) 2019. Climate change and ecosystems. Procs. Sackler Forum on Climate Change and Ecosystems, Washington, DC, November 8-9, 2018, organized by the National Academy of Sciences and The Royal Society
- ↑ Folke, C., Carpenter, S. R., Walker, B., Scheffer, M., Chapin, T. and Rockstrom, J. 2010. Resilience thinking: integrating resilience, adaptability and transformability. Ecology and Society 15(4): 20
- ↑ Scheffer, M. 2009. Critical transitions in nature and society. Princeton University Press, Princeton, New Jersey, USA
- ↑ 5.0 5.1 Brand, F.S. and K. Jax. 2007. Focusing the meaning(s) of resilience: resilience as a descriptive concept and a boundary object. Ecology and Society 12(1):23
- ↑ 6.0 6.1 6.2 Haines‐Young, R. and Potschin. M. (eds.) 2010. The Resilience of Ecosystems to Environmental Change (RECCE). Overview Report, 27 pp. Defra Project Code: NR0134
- ↑ Holling, C.S. 1973. Resilience and stability of ecological systems. Annual Rev. Ecol. Syst. 4: 1–23. doi: 10.1146/annurev.es.04.110173.000245
- ↑ Gunderson, L.H. 2000. Ecological Resilience - in Theory and Application. Annual Review of Ecology and Systematics 31:425-439.
- ↑ Hirota,M., Holmgren,M., Van Nes, E. H, and Scheffer,M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334: 232–235. doi: 10.1126/science.1210657
- ↑ Chambers, J. C., Bradley, B. A., Brown, C. S., D’Antonio, C., Germino, M. J., Grace, J. B., et al. 2014. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in the cold desert shrublands of western North America. Ecosystems 7: 360–375. doi: 10.1007/s10021-013-9725-5
- ↑ Pope, K. L., Allen, C. R., and Angeler, D. G. 2014. Fishing for resilience. T. N. Am. Fisheries Soc. 143: 467–478. doi: 10.1080/00028487.2014.880735
- ↑ Seidl, R., Spies, T. A., Peterson, D. L., Stephens, S. L., and Hick, J. A. 2016. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J. Appl. Ecol. 53 : 120–129. doi: 10.1111/1365-2664.12511
- ↑ 13.0 13.1 Dawson, T.P., Rounsevell, M.D.A., Kluvankova‐Oravska, T., Chobotova V. and Stirling, A. 2010. Dynamic properties of complex adaptive ecosystems: implications for the sustainability of services provision. Biodiversity and Conservation 19: 2843‐2853
- ↑ 14.0 14.1 14.2 Rossi, F., Vos, M. & Middelburg, J.J. 2009. Species identity, diversity and microbial carbon flow in reassembling macrobenthic communities. Oikos 118: 503-512.
- ↑ Diaz, R.J. & Rosenberg, R. 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33:245-303.
- ↑ Pimm, S.L. 1982. Food Webs. The University of Chicago Press.
- ↑ DeAngelis, D.L. 1992. Dynamics of Nutrient Cycling and Food Webs. Chapman and Hall, London.
- ↑ Vos, M., Kooi, B.W., DeAngelis, D.L. & Mooij, W.M. 2005. Inducible defenses in food webs. In: Dynamic Food Webs. Multispecies Assemblages, Ecosystem Development and Environmental Change. Eds. P.C. de Ruiter, V. Wolters & J.C. Moore. Academic Press. Pp. 114-127.
- ↑ Folke, C., Carpenter, S., Walker, B., Scheffer, M., Elmqvist, T., Gunderson, L. & Holling, C.S. 2004. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annual Review of Ecolog and Systematics 35:557-581.
- ↑ 20.0 20.1 Chambers, J.C., Allen, C.R. and Cushman, S.A. 2019. Operationalizing Ecological Resilience Concepts for Managing Species and Ecosystems at Risk. Front. Ecol. Evol. 7:241. doi: 10.3389/fevo.2019.00241
- ↑ Allen, C. R., Angeler, D. G., Cumming, G. S., Folk, C., Twidwell, D., and Uden, D. R. 2016. Quantifying spatial resilience. J. Appl. Ecol. 53, 625–635. doi: 10.1111/1365-2664.12634
- ↑ Abrams, P.A & Vos, M. 2003. Adaptation, density dependence and the responses of trophic level abundances to mortality. Evolutionary Ecology Research 5: 1113-1132
Please note that others may also have edited the contents of this article.
|