Difference between revisions of "Biogenic reefs of Europe and temporal variability"
| Line 11: | Line 11: | ||
'''''Sabellaria alveolata'''''</br> | '''''Sabellaria alveolata'''''</br> | ||
| − | Sabellaria alveolata | + | Sabellaria alveolata is a sedentary tube-dwelling polychaete closely related to Sabellaria spinulosa. They also use suspended sediment to construct their tubes, see figure 3 (Wilson, 1971<ref name= "Wilson71">WILSON D.P., 1971. ''Sabellaria'' colonies At Duckpool, North Cornwall, 1961‐1970. ''Journal of the Marine Biological Association of the UK''. '''51''', 509-580. Available form: [http://www.vliz.be/imis/imis.php?module=ref&refid=108453 www.vliz.be/imis]</ref>). In contrast to Sabellaria spinulosa, it is more commonly found in colonies. There are two major forms of colonies: veneers sand reefs ([[Natural_barriers#Biogenic reefs#Species and Characteristics#Sabellaria spinulosa | more info]].) |
The records of ''Sabellaria alveolata'' throughout Europe are greater in northern latitudes (Figure 4). This is an obvious artifact of data reporting to OBIS as ''S. alveolata'' has been reported to be widely distributed in the France, Spain and Portugal and extends as far south as Morocco (Gruet, 1982<ref name ="Gruet">GRUET Y., 1982. Recherches sur l’écologie des récifs d’Hermelles édicés par l’Annélide Polychète ''Sabellaria alveolata (Linné)'', Université des Sciences et Techniques, Nantes, France. PhD </ref>; Cunningham ''et al.'', 1984<ref name = "Cunning">CUNNINGHAM P.N., HAWKINS S.J., JONES H.D., BURROWS M.T., 1984. The geographical distribution of ''Sabellaria alveolata'' (L.) in England, Wales and Scotland, with investigations into the community structure of, and the effects of trampling on ''Sabellaria alveolata'' colonies. Report to the Nature Conservancy Council from the Department of Zoology, Manchester University, Manchester. NCC report No. HF3/11/22. </ref>). It reaches its northern limits in Britain but is restricted to the warmer waters off the west coast, as growth is inhibited below 5°C (Crisp, 1964<ref>CRISP D.J. 1964. The effects of the severe winter of 1962-63 on marine life in Britain. ''Journal of Animal Ecology.'' '''33''', 165-210.</ref>). The current confirmed northern limit is the Dumfriesshire coast of SW Scotland with records needing confirmation from the Firth of Clyde and Outer Hebrides. This species builds the largest reefs on the European coast; in particular the “Les Hermelles” reef in the Saint-Michael Bay in France, which is over 100 ha and is considered the largest reef in Europe (Gruet, 1982<ref name= "Gruet"/>; Marchand and Cazoulat, 2003 <ref>MARCHAND Y., CAZOULAT R., 2003. Biological reef survey using spot satellite data classification by cellular automata method ‐Bay of Mont Saint‐Michel (France). ''Computers & Geosciences''. '''29''', 413‐421.</ref>). | The records of ''Sabellaria alveolata'' throughout Europe are greater in northern latitudes (Figure 4). This is an obvious artifact of data reporting to OBIS as ''S. alveolata'' has been reported to be widely distributed in the France, Spain and Portugal and extends as far south as Morocco (Gruet, 1982<ref name ="Gruet">GRUET Y., 1982. Recherches sur l’écologie des récifs d’Hermelles édicés par l’Annélide Polychète ''Sabellaria alveolata (Linné)'', Université des Sciences et Techniques, Nantes, France. PhD </ref>; Cunningham ''et al.'', 1984<ref name = "Cunning">CUNNINGHAM P.N., HAWKINS S.J., JONES H.D., BURROWS M.T., 1984. The geographical distribution of ''Sabellaria alveolata'' (L.) in England, Wales and Scotland, with investigations into the community structure of, and the effects of trampling on ''Sabellaria alveolata'' colonies. Report to the Nature Conservancy Council from the Department of Zoology, Manchester University, Manchester. NCC report No. HF3/11/22. </ref>). It reaches its northern limits in Britain but is restricted to the warmer waters off the west coast, as growth is inhibited below 5°C (Crisp, 1964<ref>CRISP D.J. 1964. The effects of the severe winter of 1962-63 on marine life in Britain. ''Journal of Animal Ecology.'' '''33''', 165-210.</ref>). The current confirmed northern limit is the Dumfriesshire coast of SW Scotland with records needing confirmation from the Firth of Clyde and Outer Hebrides. This species builds the largest reefs on the European coast; in particular the “Les Hermelles” reef in the Saint-Michael Bay in France, which is over 100 ha and is considered the largest reef in Europe (Gruet, 1982<ref name= "Gruet"/>; Marchand and Cazoulat, 2003 <ref>MARCHAND Y., CAZOULAT R., 2003. Biological reef survey using spot satellite data classification by cellular automata method ‐Bay of Mont Saint‐Michel (France). ''Computers & Geosciences''. '''29''', 413‐421.</ref>). | ||
Revision as of 13:18, 24 July 2012
Contents
European-scale distribution of biogenic reefs
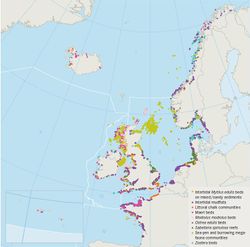
Biogenic reefs can be described as hard compact structures created by the activity of living organisms. They do not share an uniform structure and are found at variable spatial scales. Dense colonies of several species are widely considered to be reef in Europe. Only four of these species are described in this report due to their contribution to sediment entrainment, bed stability and potential wave energy attenuation, these are: Sabellaria alveolata, Sabellaria spinulosa, Modiolus modiolus and Mytilus edulis. Many biogenic reefs habitats are currently threatened and/or are in decline in Europe as a result of various natural and anthropogenic pressures (OSPAR 2010[1]). Figure 1 illustrates the distribution of some biogenic reef habitats which are currently in decline around the coast of Europe.
Sabellaria alveolata
Sabellaria alveolata is a sedentary tube-dwelling polychaete closely related to Sabellaria spinulosa. They also use suspended sediment to construct their tubes, see figure 3 (Wilson, 1971[2]). In contrast to Sabellaria spinulosa, it is more commonly found in colonies. There are two major forms of colonies: veneers sand reefs ( more info.)
The records of Sabellaria alveolata throughout Europe are greater in northern latitudes (Figure 4). This is an obvious artifact of data reporting to OBIS as S. alveolata has been reported to be widely distributed in the France, Spain and Portugal and extends as far south as Morocco (Gruet, 1982[3]; Cunningham et al., 1984[4]). It reaches its northern limits in Britain but is restricted to the warmer waters off the west coast, as growth is inhibited below 5°C (Crisp, 1964[5]). The current confirmed northern limit is the Dumfriesshire coast of SW Scotland with records needing confirmation from the Firth of Clyde and Outer Hebrides. This species builds the largest reefs on the European coast; in particular the “Les Hermelles” reef in the Saint-Michael Bay in France, which is over 100 ha and is considered the largest reef in Europe (Gruet, 1982[3]; Marchand and Cazoulat, 2003 [6]).
Sabellaria spinulosa
Sabellaria spinulosa is a tube-dwelling polychaeta (or annelid worm). It is a relatively disturbance-tolerant pioneers species (Jackson and Hiscock, 2008[7]), which mostly occurs in solitary or small aggregations. However, it can be gregarious under favorable conditions, forming large reef-structures (upto 30 cm high) (Hendrick and Foster-Smith, 2006[8]). The tubes are upright and typically consist of several layers of sediment particles( more info).

Sabellaria spinulosa reefs are known from all European coasts, except the Baltic and the waters of the Kattegat and Skagerrak, but are typically limited to areas with very high levels of suspended sediment (OSPAR 2010 [1], Figure 2). In the UK aggregations of S. spinulosa are reported to occur at a number of locations around the British Isles (Holt et al., 1998[10]; Davies et al., 2009[11]). Perhaps the best known example of an S. spinulosa reef in the UK is found in the mouth of the Wash (east coast of England), where reefs are elevated above the seafloor and have been found to extend over hundreds of square meters within the Norfolk Coast SAC (Foster‐Smith and Hendrick, 2003[12]). Relatively few records have been found in Scotland (Figure 1). Not all of these aggregations could be described as “reefs”, for instance where the species may only form superficial crusts on mixed substrata. On the German coast, intertidal and subtidal reefs have been reported from the Wadden Sea (Berghahn and Vorberg, 1993[13]) and from the southern North Sea where Linke (1951)[14] reported reefs up to 60 cm thick, 8 m wide and 60 m long. S. spinulosa has also been reported from the French coast, but without precise locations (Holt et al., 1998 [10]).
Intertidal Mytilus edulis
The distribution of Mytilus edulis (or common mussel) is circumpolar in boreal and temperate waters, in both the southern and northern hemispheres extending from the Arctic to the Mediterranean in the north‐east Atlantic (Soot‐Ryen 1955[15]). The majority of intertidal beds are found in the Wadden Sea (Netherlands, Germany and Denmark) where a 2007 inventory reported an estimated coverage of 1865 hectares in the Dutch sector (Goudswaard et al., 2007 [16]). It is also present in British coastal waters, Ireland (Jones et al., 2000 [17]) and there is a large bed (covering approximately 200 ha) in southern Brittany in France (Rollet et al., 2005 [18]).
Modiolus modiolus
Modiolus modiolus (or horse mussel) is an Arctic-boreal species that is limited in distribution by warmer temperatures to the south, but occasionally specimens have been reported as far south as Northwest Africa. It occurs from the Bay of Biscay to northern Norway, with occurrences off Iceland and the Faeroes (Tebble, 1966[19]; Poppe & Gotö, 1993[20]). It is found throughout British waters, but has most frequently been reported in northern and western areas (Figure 5). Extensive horse mussel beds are found only in parts of north and western Scotland, the Ards Peninsula, Strangford Lough, the Isle of Man, north-west Anglesey and north of the Lleyn Peninsula.
Descriptions of M. modiolus usually state the presence of aggregated clumps on mud or muddy‐gravel sediments, although the vast majority of these will not fall into the definition of biogenic reef, due to low density and coverage. However, several areas do contain large beds definable as biogenic reef including beds in Strangford Lough (Roberts, 1975), the Isle of Man (Jones, 1951; unpublished references in Holt et al., 1998[10]), Scottish waters (Comely 1978 [21]; Howson et al., 1994[22]) and within the Lleyn Peninsula (Lindenbaum et al., 2008[23]). One notable area of horse mussel beds that has received significant research are those within the Bay of Fundy on the Scotian Shelf, Canada (see Wildish et al.,2009 [24]).
Examples of temporal variability
Sabellaria alveolata
Cunningham et al. (1984)[4] reviewed the distribution and local abundance of S. alveolata in Britain. This review used past records from the literature, data from new shore surveys and reports via correspondence from other marine scientists. As a result of this exercise, changes in the extent of S. alveolata distribution over a period of approximately 100 years were documented. In order to evaluate the long-term temporal variability in S. alveolata distribution and abundance, the data were divided into three arbitrary periods: pre-1963 (before the cold winter of 1962/1963), 1964-1979 and 1980-1984 (Cunningham et al., 1984[4]).
Frost et al. (2005)[25] carried out a series of broadscale and focused mapping studies of S. alveolata in NW England and North Wales in 2003/04. This comprised a resurvey of sites that had been previously surveyed in the 1980s (Cunningham et al. 1984[4]). S. alveolata was found to be present at most of the sites where it had previously been recorded (e.g. Cunningham, 1984[4]) and at many of these sites it appears also to have increased in abundance (Table 1). S. alveolata had re-appeared in areas where it has been absent for many years (Table 1: Hilbre Island and Colwyn Bay) and had spread to areas for which there are no known previous records (Table 1: North Wirral, Rossal Point).
Hawkins (1993) suggested that S. alveolata was declining along the Cumbrian coast, but the present study found it to be abundant or super‐abundant at most sites. The records from the present study therefore seem to confirm the observation made by others that S. alveolata shows a great deal of temporal variability within a fairly constant geographic range (e.g. Cunningham et. al., 1984[4]). Even on a shore where S. alveolata is continually present, there is a great deal of variability in terms of abundance and ‘within shore’ distribution. For example, long term studies at Duckpool in North Cornwall (Wilson 1971[2]; 1974[26]; 1976[27]) and in Normandy, France (Gruet, 1986[28]) have revealed a great deal of variability over the years in the distribution and abundance of S. alveolata colonies within sites.
| Location | S. alveolata abundance | |||
|---|---|---|---|---|
| Pre-1963 | 1964-1979 | 1980-1984 | 2003-2004 | |
| Penmon | N | N | ||
| Great Orme’s Head | N | N | ||
| Little Orme’s Head | N | N | ||
| Rhos-on-Sea | N | N | ||
| Colwyn Bay | P | N | R | |
| Hilbre Island | A | R | N | A |
| Wirral Foreshore | A | |||
| Lytham Pier | N | N | ||
| St Annes Pier | N | N | ||
| Fleetwood,Rossall Pt | N | F | ||
| Heysham* | F-O | N | N | |
| Holme Island | N | N | ||
| Humphrey Head | N | N | ||
| Wadhead, Scar | N | N | ||
| Walney Island | N | N | ||
| Annaside Bank | A | SA | ||
| Tarn Bay | A-SA | SA | ||
| Drigg | A | SA | ||
| Seascale | O | SA | ||
| Sellafield | O | A-SA | ||
| Nethertown | A | A | ||
| St. Bees | O | C-A | ||

Sabellaria spinulosa
Subtidal S. spinulosa reefs have been reported to have been lost in at least five areas of the northeast Atlantic (Jones et al., 2000[17]). During the 1920s large reefs of S. spinulosa were common in the German Wadden Sea (Hagmeier and Kändler, 1927[29]) but most have since been lost. Similar records of loss have been recorded from the Lister Ley (Island of Sylt) and the Norderau area (Riesen and Reise, 1982[30]; Reise and Schubert, 1987[31]). Only three living reefs were found during surveys in the early 1990s compared to 24 during the 19th century (Figure 6). In the late 1990s, samples taken from the subtidal reefs in the German Wadden Sea consisted largely of compact lumps of empty tubes. In 2000, one of these reefs had diminished drastically in extent with the remainder in poor condition although dredge samples were occupied by many tiny tubes with living worms inside. A third reef which had previously extended over ~18 hectares could not be located during repeat surveys in 2002. In the UK there are reports of reefs being lost in Morecambe Bay (Taylor and Parker, 1993[32]), the Wash and the Thames (Warren and Sheldon, 1967[33]). In the western North Sea report comparing records from 1986 and 2000 suggest an increase in distribution and densities in the western North Sea (Rees, 2007[34]).
Modiolus modiolus
Only a few beds are known have been surveyed over long enough time spans for evidence of change to be apparent. In the Irish Sea, south of the Isle of Man, an extensive bed was almost completely lost due to scallop dredging (Veale et al., 2000[35]). For similar reasons, beds in Strangford Lough (Northern Ireland) also showed severe declines (Service and Magorrian, 1997[36]). Recently, beds in North Anglesey (Wales) have been destroyed by fishing activity (Holt, 2008[37], Countryside Council for Wales, pers. comm.). By contrast, in an Icelandic bay Modiolus modiolus was still the dominant by‐catch species in scallop dredges 30 years after scallop dredging began (Garcia and Ragnarsson, 2007[38]). In Sullom Voe (Shetland) a bed coincident with a pipeline showed signs of recovery, with some re‐colonisation of disturbed sediment after a few years (Mair et al. 2000[39]). On the legs of an oil platform in the North Sea a substantial population was present 10 years after installation, but in this situation the young mussels would have been free of much predation (Anwar et al. 1990[40]). As a species it appears to have declined in the North Sea. Comparing occurrences by ICES Rectangles Callaway et al. (2007)[41] showed that the species had been found in 11 rectangles in the 1982‐85 period, but comparable international surveys in 2000 found it in only 1 rectangle.
Mytilus edulis
Surveys covering the whole littoral of Niedersachsen, in Germany, revealed a decrease in the extent of M. edulis (5000 hectares in the late 1950s, 2700 ha in 1989/91, 1300 ha in 1994 to 170 ha in 1996). Mussel beds in the Ameland region have also disappeared after intensive fishing in the region (Dankers 1993[42]). In the Netherlands, Higler et al. (1998[43]) observed a serious decline in the populations of mussels between 1988 and 1990, mainly caused by fisheries. The extent of mussel beds decreased from the 1970s to the 1990s. In Denmark, intensive fisheries during 1984 to 1987 almost led to a complete disappearance of the mussel population (Kristensen, 1995[44]).
See also
Natural barriers_ Biogenic reefs
Dynamics, threats and management of biogenic reefs action
References
- ↑ 1.0 1.1 1.2 1.3 OSPAR, 2010. Quality Status Report 2010. OSPAR Commission. London. 176 pp. Available from: www.vliz.be/imis
- ↑ 2.0 2.1 WILSON D.P., 1971. Sabellaria colonies At Duckpool, North Cornwall, 1961‐1970. Journal of the Marine Biological Association of the UK. 51, 509-580. Available form: www.vliz.be/imis
- ↑ 3.0 3.1 GRUET Y., 1982. Recherches sur l’écologie des récifs d’Hermelles édicés par l’Annélide Polychète Sabellaria alveolata (Linné), Université des Sciences et Techniques, Nantes, France. PhD
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 CUNNINGHAM P.N., HAWKINS S.J., JONES H.D., BURROWS M.T., 1984. The geographical distribution of Sabellaria alveolata (L.) in England, Wales and Scotland, with investigations into the community structure of, and the effects of trampling on Sabellaria alveolata colonies. Report to the Nature Conservancy Council from the Department of Zoology, Manchester University, Manchester. NCC report No. HF3/11/22.
- ↑ CRISP D.J. 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology. 33, 165-210.
- ↑ MARCHAND Y., CAZOULAT R., 2003. Biological reef survey using spot satellite data classification by cellular automata method ‐Bay of Mont Saint‐Michel (France). Computers & Geosciences. 29, 413‐421.
- ↑ ckson, A., Hiscock, K., 2008. Sabellaria spinulosa. Ross worm. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom. [cited 28/04/2010]. Available from:www.marlin.ac.uk
- ↑ Hendrick, V.J., Foster-Smith, R.L., 2006. Sabellaria spinulosa reef: a scoring system for evaluating 'reefiness' in the context of the Habitats Directive. Journal of the Marine Biological Association of the United Kingdom. 86, 665-677.
- ↑ worms-website
- ↑ 10.0 10.1 10.2 HOLT T.J., REES E.I., HAWKINS, S.J., SEED, R., 1998. Biogenic Reefs (volume IX). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scottish Association for Marine Science (UK Marine SACs Project). 170 pp. Available from: www.vliz.be/imis
- ↑ DAVIES A.J., LAST K.S., ATTARD K., HENDRICK V.J., 2009. Maintaining turbidity and current flow in laboratory aquarium studies, a case study using Sabellaria spinulosa. Journal of Experimental Marine Biology and Ecology. 370, 35-40.
- ↑ FOSTER‐SMITH R.L., HENDRICK V.J., 2003. Sabellaria spinulosa reef in The Wash and North Norfolk cSAC and its approaches: Part III, Summary of knowledge, recommended monitoring strategies and outstanding research requirements. English Nature Research Reports Number 543.
- ↑ BERGHAHN R., VORBERG R., 1993. Effects of the shrimp fisheries in the Wadden Sea. In: Influence of fisheries upon Marine Ecosystems. Einfluss Der Fischerei Auf Marine Oekosysteme Lukowicz, M., 103-126.
- ↑ LINKE O., 1951. Neue Beobachtungen uber Sandkorallen‐Riffe in der Nordsee, Natur u. Volk. 81, 77‐84.
- ↑ SOOT‐RYEN T., 1955. A report on the family Mytilidae. Allan Hancock Pacific Expedition. 20, 1-154.
- ↑ GOUDSWAARD P.C., JANSEN J.M.J., VAN ZWEEDEN C., KESTELOO J.J., VAN STRAALEN M.R., 2007. Het mosselbestand en het areaal aan mosselbanken op de droogvallende platen in de Waddenzee in het voorjaar van 2007. Wageningen IMARES, December 2007. Available from: www.vliz.be/imis
- ↑ 17.0 17.1 JONES L.A., HISCOCK K., CONNOR D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report).
- ↑ ROLLET C., BONNOT-COURTOIS C., FOURNIER J., 2005. Cartographie des habitats benthiques médiolittoraux à partir des orthophotographies littorales. Fiche technique-Projet REBENT FT13-2005-01, Ifremer, Brest. 18pp.
- ↑ TEBBLE N., 1966. British bivalve seashells. Natural History Museum, London. pp 212.
- ↑ POPPE G., GOTO Y., 1993. European seashells. Volume:2 (Scaphopoda, Bivalvia, Cephalopoda). Conchbooks, Haekenheim. 221 pp. Available from: www.vliz.be/imis
- ↑ COMELY C.A. 1978. Modiolus modiolus (L.) from the Scottish west coast. Ophelia. 17, 167‐193.
- ↑ HOWSON C., CONNOR D., HOLT R., 1994. The Scottish sealochs - an account of surveys undertaken for the Marine Nature Conservation Review. Joint Nature Conservation Committee Report, No. 164.
- ↑ LINDENBAUM C., BENNELL J., REES E., MCCLEAN D., COOK W., WHEELER A., SANDERSON W., 2008. Small-scale variation within a Modiolus modiolus (Mollusca: Bivalvia) reef in the Irish Sea: I. Seabed mapping and reef morphology. Journal of the Marine Biological Association of the UK. 88, 133-141.
- ↑ WILDISH D.J., FADER G. & PARROTT D., 2009. A model of horse mussel reef formation in the Bay of Fundy based on population growth and geological processes. Atlantic Geology. 45, 157 170.
- ↑ 25.0 25.1 FROST M.T., LEAPER R., MIESZKOWSKA N., MOSCHELLA P., MURUA J., SMYTH C., HAWKINS S.J., 2005. Recovery of a Biodiversity Action Plan Species in Northwest England: possible role of climate change, artificial habitat and water quality amelioration. A report submitted to English Nature, spring 2004.
- ↑ WILSON D.P., 1974. Sabellaria Colonies at Duckpool, North Cornwall, 1971–1972, With a Note for May 1973. Journal of the Marine Biological Association of the United Kingdom. 54, 393-436.
- ↑ WILSON D.P., 1976. Sabellaria Alveolata (L.) At Duckpool, North Cornwall, 1975. Journal of the Marine Biological Association of the United Kingdom. 56, 305-310.
- ↑ GRUET Y., 1986. Spatio‐temporal changes of Sabellarian reefs built by the sedentary polychaete Sabellaria alveolata (Linn6) P.S.Z.N.I. Mar. Ecol. 7(4), 303‐319.
- ↑ HAGMEIER A., KANDLER R., 1927. Neue Untersuchungen im nordfriesischen Wattenmeer und auf den fiskalischen Austernbanken.-Wiss. Meeresunters. (Abt. Helgoland). 16, 1-90.
- ↑ RIESEN W., REISE K., 1982. Macrobenthos of the subtidal Wadden Sea: Revisited after 55 years, Helgolander Meeresuntersuchungen. 35, 409‐423.
- ↑ REISE K., SCHUBERT A., 1987. Macrobenthic turnover in the subtidal Wadden Sea: The Norderaue revisited after 60 years. Helgolander Meeresuntersuchungen. 41, 69-82.
- ↑ TAYLOR P.M., PARKER J.G., 1993. An Environmental Appraisal: The Coast of North Wales and North West England, Hamilton Oil Company Ltd, 80 pp.
- ↑ WARREN P.J., SHELDON R.W., 1967. Feeding and migration patterns of the Pink Shrimp Pandalus montagui, in the estuary of the River Crouch, England. Journal of the Fisheries Research Board of Canada. 24, 569-580.
- ↑ REES, H.L.; EGGLETON, J.D.; RACHOR, E.; VANDEN BERGHE, E. (Ed.) (2007).Structure and dynamics of the North Sea benthos. ICES Cooperative Research Report, 288. ICES: Copenhagen. ISBN 87-7482-058-3. III, 258 + annexes pp. Available from: www.vliz.be/imis
- ↑ VEALE L.O., HILL A.S., HAWKINS S.J., BRAND A.R., 2000. Effects of long-term physical disturbances by commercial scallop fishing on subtidal epifaunal assemblages and habitats. Marine Biology. 137, 325-337.
- ↑ SERVICE M., MAGORRIAN B. H., 1997. The extent and temporal variation of disturbance of epibenthic communities in Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom. 77, 1151-1164.
- ↑ HOLT 2008, Countryside Council for Wales, pers. comm.
- ↑ GARCIA, E. G., & RAGNARSSON, S. A. 2007. Impact of scallop dredging on macrobenthic communities in Breidafjordur, West Iceland. In: GARCIA, E. G., RAGNARSSON, S.A,, STEINGRIMSSON S. A, NAEVESTADD., HARALDSON H. P., FOSSA J. H., TENDAL, O. S,, & ERIKSSON H. (eds) Bottom Trawling and Scallop Dredging in the Arctic: Impacts of fishing on non‐target species, vulnerable habitats and cultural heritage. Nordic Council of Ministers, Copenhagen, Chapter 2.2.
- ↑ MAIR J. M., MOORE C. G., KINGSTON P. F. & HARRIES D. B., 2000. A review of the status, ecology and conservation of horse mussel Modiolus modiolus beds in Scotland. Scottish Natural Heritage, Edinburgh (Commissioned Report F99PA08).
- ↑ ANWAR N. A., RICHARDSON C.A., & SEED R., 1990. Age determination, growth rate and population structure of the horse mussel Modiolus modiolus. Journal of the Marine Biological Association of the United Kingdom. 70, 441‐457.
- ↑ CALLAWAY R., ENGELHARD G. H., DANN J, COTTER J., & RUMHOR H., 2007. A century of North Sea epibenthos and trawling comparisons between 1902‐1912, 1982-1895 and 2000. Marine Ecology Progress Series. 346, 27-43.
- ↑ DANKERS N., 1993. Integrated estuarine management-obtaining a sustainable yield of bivalve resources while maintaining environmental quality. In: DAME R. R. (ed) Bivalve filter feeders in estuarine and ecosystem processes. Springer, Berlin, 479-511. Available form: www.vliz.be/imis
- ↑ HIGLER B., DANKERS N., SMAAL A.,DE JONGE V.N., 1998. Evaluatie van de ecologische effecten van het reguleren van schlpdievisserij in Waddenzee en Delta op bodemorganismen en vogels. In: VAN DIJK J.J. and R. HEILING (eds.) Structuurnota Zee- en Kustvisserij, van de maatregelen in de kustvisserij gedurende de eerste fase (1993–1997). Appendix 5, pp. 17.
- ↑ KRISTENSEN P.S., 1995. Aerial surveys, biomass estimates, and elimination of the mussel population (Mytilus edulis L.), in the Danish Wadden Sea, 1991±1994. ICES C.M. 1995/K:44, 22 pp. Available from:www.vliz.be/imis
Please note that others may also have edited the contents of this article.
|