Dynamics, threats and management of biogenic reefs
UNDER CONSTRUCTION
Contents
PROCESSES AND MECHANISMS DRIVING NATURAL DYNAMICS & ECOSYSTEM DEVELOPMENT
Biogenic reefs can be described as hard compact structures created by the activity of living organisms [1]. They do not share an uniform structure[1] and vary in spatial scale. Moreover, the life they support is greatly dependent upon location and composition[2]. Dense colonies of several species are widely considered to be reef in Europe. Only four of these species are described in this report due to their contribution to sediment entrainment, bed stability and potential wave energy attenuation, these are: Sabellaria spinulosa, Sabellaria alveolata, Mytilius spp. and Modiolus modiolus[1]. In this section, the processes and mechanisms driving natural dynamics and ecosystem development of biogenic reefs are discussed for each group in turn.
Sabellaria spinulosa
Environmental Requirements
S. spinulosa is thought to require stable foundations on which to settle and establish a tube (Jackson, 1977[3]; Wood, 1999[4]; Chisholm and Kelley, 2001[5]) and is thus likely to favour substrata which include bedrock; boulders, cobbles, mixed substrata; and mixed sediment (Connor et al., 1997[6]). Although it is assumed that a firm substratum is required for colony establishment, it has been suggested that a reef can increase in extent without the need for hard substratum (Holt et al., 1997[7]). Many studies have reported extensive colonies in predominantly sandy areas (Warren and Sheldon, 1967[8]; Schäfer, 1972[9]; Warren, 1973[10]; Limpenny et al., 2010[11]). Recent observations from The Wash, England show that S. spinulosa had ‘seeded’ on shell fragments predominantly from blue or horse mussels (Ian Reach, Natural England, pers. comm.).
As S. spinulosa is a sedentary species, it relies on wave and current action to supply food and wash away waste products (Kirtley, 1992[12]). Strong water movement is required for food provisions, but is perhaps more important to raise sediment into suspension for tube building (Jones, 1999[13]). As a result, S. spinulosa colonies are typically located in areas of weak to moderately strong water flow (Jones et al., 2000[14]). It also appears to favour locations around the edges of sand banks or areas with sand waves (Foster‐Smith, 2001[15]). S. spinulosa typically occurs subtidally in depths of a few meters to up to 40 m depth (Caspers, 1950[16]; George and Warwick[17], 1985; Connor et al., 1997[6]; Jessop and Stoutt, 2006[18]), but can occur in depths up to 600 m (Hartmann-Schröder, 1971). S. spinulosa occasionally occurs in the lower intertidal zone (Jessop and Stoutt, 2006[18]).
Reproduction and Development
The fecundity and recruitment of S. spinulosa is known to be variable (e.g. Linke, 1951[19]; Wilson, 1971[20]; Michaelis, 1978[21]; George and Warwick, 1985[17]). The family Sabellariidae are broadcast spawners, reproducing sexually, resulting in larvae that drift passively in the plankton (Schäfer, 1972[9]; Eckelbarger, 1978[22]). The larvae can spend a few weeks to several months in the plankton (Wilson, 1929[23]) before seeking appropriate conditions for settlement (Wilson, 1968[24]; Eckelbarger, 1978[22]). If conditions are unsuitable, the larvae are able to delay metamorphosis for several weeks. Physical factors alone have limited influence on settlement (Wilson, 1968[24]) and settlement and metamorphosis is strongly influenced by the tube cement of other sabellariids (Wilson, 1968[24]; 1970[25]; Eckelbarger, 1978[22]; Jensen, 1992[26]). This mechanism ensures settlement in a suitable habitat and promotes the development of large colonies.
Despite only a few studies investigating the rate at which S. spinulosa can extend their dwelling tubes (Hendrick, 2007[27]; Davies et al., 2009[28] being exceptions), it appears that sabellariid reefs develop quickly following successful settlement (Linke, 1951[19]; Vorberg, 2000[29]; Stewart et al., 2004[30]; Braithwaite et al., 2006[31]). Last et al. (2011)[32] observed that tube extension rates are highly variable and that they could grow up to 6 mm a day for several days when provided with an adequate sediment supply.
Little is known about the longevity of S. spinulosa colonies, but sabellariids are expected to survive for 1-2 years (Kirtley, 1966[33]; McCarthy, 2001[34]; McCarthy et al., 2003[35]), with some reports of longer life spans (Wilson, 1974[36]; George and Warwick, 1985[17]). It is likely that the age of an actual colony may greatly exceed the age of the oldest individuals. This is particularly likely as sabellariid larvae are stimulated to metamorphose by conspecific secretions, encouraging continuous succession of generations.
Sabellaria alveolata
Environmental Requirements
S. alveolata generally requires hard substrata on which to develop, but these must be in areas with a good supply of suspended coarse sediment for tube building. S. alveolata reefs are known to form on a range of substrata from pebble to bedrock (Cunningham et al., 1984[37]). Reefs therefore commonly form on bodies of rock or boulders surrounded by sand. Larsonneur (1994)[38] noted that settlement of S. alveolata was facilitated by the sand mason Lanice conchilega which can stabilize sand well enough to allow colonization by S. alveolata. Settlement occurs mainly on existing colonies or their dead remains (Figure 1).
Water movement of sufficient intensity is a prime requirement to suspend coarse sand particles, thus making them available for the building of worm tubes. Cunningham et al. (1984)[37] note that this may consist of waves or currents. In many British localities such as the south west of England, much of Wales and the Cumbrian coast, the former seem more important. In other areas, such as parts of the Severn Estuary, tidal suspension is probably very important. However, S. alveolata is generally absent in very exposed peninsulas such as the Lleyn, Pembrokeshire and the extreme south west of Cornwall, which probably relates to the effect of water movement on recruitment (Cunningham et al., 1984[37]).
Reproduction and Development
It is thought that the larvae of S. alveolata spend 6 weeks to 6 months in the plankton (Wilson, 1968[24]; Wilson, 1971[20]) in order to attain widespread dispersal. The most detailed work done on S. alveolata reproduction in the British Isles is that of Wilson in Cornwall (e.g. Wilson, 1971[20]). Wilson observed slight settlement in every month except July, but in 14 years of monitoring (1961 to 1975), Wilson (1976)[39] observed only three heavy settlements: in 1966, 1970 and 1975. All occurred from September to November or December. Subsequent studies have revealed that the intensity of settlement is extremely variable, both temporally and spatially (Gruet, 1982[40]; Cunningham et al., 1984[37]). Settlement occurs mainly on existing colonies or their dead remains; chemical stimulation seems to be involved, and this can come from S. spinulosa tubes as well as from S. alveolata (Wilson, 1971[20]; Gruet, 1982[40]; Cunningham et al., 1984[37]).
Mytilus spp.
Environmental Requirements
The widespread distribution of the M. edulis is a reflection of its tolerance of a wide range of environmental variables. Natural reefs typically occur on firm, mixed sediments in relatively wave sheltered estuaries and bays characterized by strong currents (Holt et al., 1998[41]). In more exposed areas, larger colonies are only able to develop on hard and stable substrata such as rock or large boulders (Seed, 1969[42]). Conversely, in sheltered environments large beds may develop on more sandy substrates (Roberts and McKenzie, 1983[43]).
Mussels produce byssal threads which anchor them to the substratum and each other, enabling large beds to develop. Mussels can grow in all but the most exposed conditions where their byssus threads can provide anchorage against wave action and water flow. As M. edulis is a sessile filter feeder, it requires sufficient water to flow to bring food and wash away waste. Larger beds require higher flow in order to provide sufficient food supply to high numbers of individuals. It is generally considered that this water movement is best provided by tidal currents rather than wave action, though the latter may also contribute in some areas (Holt et al., 1998[41]).
M. edulis is tolerant of a wide range of salinities, being found in locations ranging from estuarine to fully marine, but larger reefs typically occur within the lower third of the intertidal and in the mid to lower reaches of the estuary (Holt et al., 1998[41]). M. edulis reefs do form subtidally and have been reported to occur at depths of 30 m (Ian Reach, Natural England, pers. comm.). The upper limits of M. edulis are thought to be set by temperature and desiccations stress (Seed and Suchanek, 1992[44]) in addition to reduced feeding (Widdows and Shick, 1985[45]). The lower limits are generally set by biological factors such as competition and predation with physical factors playing a secondary role (Holt et al., 1998[41]).
Reproduction and Development
The M. edulis fecundity and recruitment success is highly variable, both temporally and spatially. It can reproduce in its first year and can spawn throughout the year, with a major spawning event usually occurring in the spring (Seed, 1969[42]). Larvae can survive in the plankton for 2‐4 weeks before metamorphosis, although this can be up to 6 months, depending on availability of food, suitable substrate and temperature (Holt et al., 1998[41]). Settlement can be either a one-stage or a two‐stage process. Some larvae can settle directly onto adult beds (McGrath et al., 1988[46]) or they can temporarily settle onto sublittoral filamentous substrata such as algae or hydroids before becoming detached, and eventually settling onto an adult bed (Bayne, 1964; Pulfrich, 1996[47]). It is thought that this may be a mechanism for reducing competition between very young and adult mussels, and/or to prevent filtration of the larvae by the adult mussels. McGrath et al. (1988)[46] reported very large densities of settling spat in Ireland, but more commonly modest recruitment between the shells of adult mussels provides sufficient supply to maintain persistent beds (Holt et al., 1998[41]). Conversely, heavy recruitment may not necessarily lead to the formation or maintenance of a dense bed or reef if predation or losses due to wave action are high.
M.edulis growth and production can be extremely high, particularly in sheltered or estuarine areas (Holt et al., 1998). It has been reported that M. edulis accounts for 20% of the total macrobenthic production in the Wadden Sea (Beukema , 1981[48]), whilst Dare (1976)[49] estimated the production by two year classes to be 2.5‐3 times their maximum standing crop, with few mussels surviving beyond their third year. It is thought that the majority of mussels do not survive beyond 3 years of age (Seed, 1976[50]), there are reports of individuals surviving beyond 15 years (Sukhotin et al., 2007[51]).
Modiolus modiolus
Environmental Requirements
Despite typically occurring on hard substrata, M. modiolus beds and reefs are capable of forming on a variety of sedimentary bottoms, ranging from muddy substrata in some sea lochs to quite coarse mixed sediments containing much stones and shell. Larvae can also settle on artificial substrates such as oil rigs and can form reefs on these structures. The byssus threads of adult M. modiolus provide a suitable substrate for attachment and protection from predators. Beds occurring infaunally can lack available byssus threads and thus limit the recruitment (Holt and Shalla, 1997[52]) and the development of larger beds.
M. modiolus has a very wide depth distribution, typically being found subtidally from a few meters of depth right down to depths of 280 m (Schweinitz and Lutz, 1976[53]). Intertidal populations have occasionally been reported (Davenport and Kjosvik, 1982[54]), but these are thought to be limited by temperature and desiccation stress associated with aerial exposure (Coleman, 1976[55]; Davenport and Kjosvik, 1982[54]). The densest populations that are known as reef are found between 5 and 50 m in British waters (Holt et al., 1998[41]), whilst infaunal reefs have been found at over 80 m in the Bay of Fundy (Wildish et al., 2009[56]).
Reproduction and Development
M. modiolus is a long-lived species with individuals only reaching sexual maturity between 3 and 6 years of age. It is thought that this adaptation is in response to high predation on juvenile mussels, thereby channeling energetic resources towards growth in early life. As a result, M. modiolus exhibits rapid growth in the first few years of life, followed by much slower growth following sexual maturation (Anwar et al., 1990[57]). M. modiolus spawning is known to be variable, both temporally and spatially. In Strangford Lough, Northern Ireland, slight spawning is known to occur year-round, with no apparent peak (Seed and Brown, 1977[58]; Brown, 1984[59]). Conversely, in Scandinavia, a spawning peak occurs in June, followed by a period of gonad redevelopment. Spawning is temperature dependent and is reported to occur within a narrow temperature range (7-10 °C). It is thought that the relatively constant temperatures in Strangford Lough facilitate the year-round spawning (Brown, 1984[59]). M. modiolus in the Irish Sea off the SE coast of the Isle of Man has been observed to follow an annual cycle of gonad development with a peak occurring in spring/summer, with trickle spawning occurring all year round (Jasim and Brand, 1989[60]).
VULNERABILITY & THREATS
GENERAL SUMMARY
This section is divided up into (1) the vulnerability and (2) the threats (biological, chemical and physical) to each species in turn: Sabellaria spinulosa; Sabellaria alveolata; Mytilus spp. and Modiolus modiolus.
In this section, we refer to the sensitivity, vulnerability and potential for recovery of the habitat to sea level rise and storm events. In the case of natural reefs, flooding is not applicable and is therefore not discussed here. Much of the information from this section was sourced from the Marine Life Information Network website ([26]). We have adopted the terminology used by MarLIN with definitions below. In the following sections, we have identified the factors that are most likely to be associated with sea level rise and storm events for each species. The ‘intolerance’, ‘sensitivity’ and ‘recoverability’ of each species are presented in table format.
Intolerance is the susceptibility of a habitat, community or species (i.e. the components of a biotope) to damage, or death, from an external factor. Intolerance must be assessed relative to change in a specific factor.
Recoverability is the ability of a habitat, community, or species (i.e. the components of a biotope) to return to a state close to that which existed before the activity or event caused change.
Sensitivity is dependent on the intolerance of a species or habitat to damage from an external factor and the time taken for its subsequent recovery. For example, a very sensitive species or habitat is one that is very adversely affected by an external factor arising from human activities or natural events (killed/destroyed, 'high' intolerance) and is expected to recover over a very long period of time, i.e. >10 or up to 25 years ('low'; recoverability). Intolerance and hence sensitivity must be assessed relative to change in a specific factor.
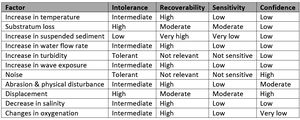
Sabellaria spinulosa
S. spinulosa is generally considered to be a very tolerant species with limited sensitivity (Table 1). Perhaps, the greatest sensitivity is to substratum loss, as once dislodged, the individual worms cannot rebuild their tubes. S. spinulosa is often one of the first species to recolonise an area after a disturbance (Cooper et al., 2007). Therefore, this species is expected to have a high recoverability.
S.spinulosa is most frequently found in polluted and disturbed conditions. S. spinulosa occurs in high densities on subtidal gravels that would be expected to be disturbed every year or perhaps once every few years due to storms and in polluted conditions. S. spinulosa appears to be very tolerant of water quality variation, but is potentially vulnerable to the short‐term and localized effects of mineral extraction and the effects of oil dispersants on the larvae.
| Factor | Intolerance | Recoverability | Sensitivity | Confidence |
|---|---|---|---|---|
| Increase in temperature | Low | High | Low | Very low |
| Substratum loss | High | High | Moderate | High |
| Increase in suspended sediment | Low | Immediate | Not sensitive | Moderate |
| Increase in water flow rate | Intermediate | High | Low | Moderate |
| Increase in turbidity | Tolerant | Not relevant | Not sensitive | Low |
| Increase in wave exposure | Intermediate | High | Low | Moderate |
| Noise | Tolerant | Not relevant | Not sensitive | Low |
| Abrasion & physical disturbance | Intermediate | High | Low | Low |
| Displacement | High | High | Moderate | Low |
| Decrease in salinity | Intermediate | High | Low | Moderate |
| Changes in oxygenation | Intermediate | High | Low | Very low |
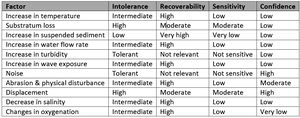
Sabellaria alveolata
Similar to S. spinulosa, recolonisation of individual S. alveolata is expected to be high, as long as there is suitable substratum for the settlement of larvae (Table 2). Recovery of reefs is expected to take considerably longer.
| Factor | Intolerance | Recoverability | Sensitivity | Confidence |
|---|---|---|---|---|
| Increase in temperature | Intermediate | High | Low | low |
| Substratum loss | High | Moderate | Moderate | Low |
| Increase in suspended sediment | Low | Very high | Very low | Low |
| Increase in water flow rate | Intermediate | High | Low | Low |
| Increase in turbidity | Tolerant | Not relevant | Not sensitive | Low |
| Increase in wave exposure | Intermediate | High | Low | Low |
| Noise | Tolerant | Not relevant | Not sensitive | High |
| Abrasion & physical disturbance | Intermediate | High | Low | Moderate |
| Displacement | High | Moderate | Moderate | High |
| Decrease in salinity | Intermediate | High | Low | Low |
| Changes in oxygenation | Intermediate | High | Low | Very low |
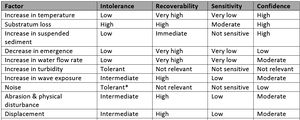
Mytilus spp.
Seed and Suchanek (1992)[44] suggested that although mussel assemblages found in the upper intertidal or most sheltered sites experience the least change per unit time and may be considered more 'stable' (Lewis, 1977[62]), these assemblages would recover much slower than lower intertidal and more exposed sites if disturbed. In addition, Mytilus spp. recovers quicker than other Mytilus species (Seed and Suchanek, 1992[44]). Overall, Mytilus spp. populations are considered to have a strong ability to recover from environmental disturbances (Table 3, Holt et al., 1998[41]; Seed and Suchanek, 1992). Larval supply and settlement could potentially occur annually, but settlement is sporadic with unpredictable pulses of recruitment (Lutz and Kennish, 1992[63]; Seed and Suchanek, 1992[44]). Therefore, while good annual recruitment is possible, recovery may take at least 5 years, although in certain circumstances and under some environmental conditions, recovery may take significantly longer (Tyler‐Walters, 2008[64]).
| Factor | Intolerance | Recoverability | Sensitivity | Confidence |
|---|---|---|---|---|
| Increase in temperature | Low | Very high | Very low | High |
| Substratum loss | High | High | Moderate | High |
| Increase in suspended sediment | Low | Immediate | Not sensitive | High |
| Decrease in emergence | Low | Very high | Very low | Low |
| Increase in water flow rate | Low | Very high | Very low | Moderate |
| Increase in turbidity | Tolerant | Not relevant | Not sensitive | Not relevant |
| Increase in wave exposure | Intermediate | High | Low | Moderate |
| Noise | Tolerant* | Not relevant | Not sensitive | Low |
| Abrasion & physical disturbance | Intermediate | High | Low | Moderate |
| Displacement | Intermediate | High | Low | Moderate |
| Decrease in salinity | Low | Very high | Very low | Moderate |
| Changes in oxygenation | Low | Very high | Very low | High |
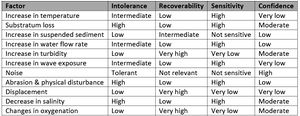
Modiolus modiolus
M. modiolus is a long‐lived species and individuals are commonly observed to be older than 25 years. This species is regarded to be intolerant of loss of substratum, physical damage and abrasion (Table 4). Recovery is thought to take many years due to sporadic recruitment (Tyler-Walters, 2007[65]).
M. modiolus individuals or reefs are generally not considered to be fragile, however, physical threats from fishing gears pose a significant threat to this species. Older individuals are susceptible to boring by the sponge Clione celata which can make shells brittle, thus increasing vulnerability (Comely, 1978[66]).
NATURAL AND ANTHROPOGENIC THREAT
These organisms are exposed to a broad range of threats; therefore not all are discussed in this section. The most severe threats have been given priority (physical, chemical and biological), with particular emphasis on those relating to floods and storms.
Physical threats can originate from natural and anthropogenic sources. Natural sources include increased temperatures, an increase in storm occurrence and intensity and sea‐level rise, all of which occurre as a result of global climate change. In this section we holistically address the general physical pressures each species faces, rather than those from individual processes. Physical anthropogenic threats to reefs are extensive, so not all are covered in this document. Some of the major threats to natural reefs are the impact of fishing gears, marine aggregate extraction, coastal development (including the construction of coastal defences), construction of offshore marine renewable and oil and gas exploration. Natural chemical threats posed by climate change include reduced salinity, brought about by increased precipitation and surface runoff, and acidification brought about by reduced pH and changes in oxygen concentrations. Anthropogenic chemical threats are primarily those associated with pollution. Biological threats are usually considered to be natural in the form of parasites, predators and competitors. However, invasion by non‐native species is often a result of human introduction and therefore can indirectly be considered an anthropogenic threat.
Sabellaria spinulosa
Physical threats
Sabellaria spinulosa usually occurs subtidally in areas of high water flow, and is relatively tolerant of wave and tidal‐forcing. However, as S. spinulosa generally grows upon cobbles and pebbles (Connor et al., 2004[67]), and since it has been suggested that an increase in wave or tidal flow may reduce the stability of the attachment substratum, this can result in increased scouring and mortality of individuals (Jackson and Hiscock, 2008[68]). It is a relatively disturbance‐tolerant species and is often the first species to recolonise an area after a physical disturbance (Jackson and Hiscock, 2008[68]). The physical disturbance of removal from tubes and substratum loss will cause mortality. As S. spinulosa is predominantly subtidal, it is likely to be less affected by temperature changes than the intertidal S. alveolata, which has been shown to be severely affected by low winter temperatures (Crisp, 1964[69]). Fisheries for the pink shrimp (Pandalus montagui) and brown shrimps (Crangon crangon) (often associated with areas of Sabellaria spinulosa reefs) have been implicated in the loss or damage of reefs. However, Vorberg (2000)[29] undertook experimental and observational studies that indicated only minor damage to tubes and rapid recovery as a result of shrimp fisheries. Nevertheless, populations, especially loose aggregations, may be displaced by mobile fishing gear.
Chemical threats
There is little data available on chemical threats to S. spinulosa, although it is not thought to be sensitive to reduced salinity (Jackson and Hiscock, 2008[68]).
Biological threats
There is insufficient information available on biological threats to S. spinulosa.
Sabellaria alveolata
Physical threats
Sabellaria alveolata is typically found in the intertidal and is tolerant of changes in sediment regime. The physical disturbance of removal from tubes and substratum loss will cause mortality. Being an intertidal species, the greatest threats come from cold air temperatures and heavy wave action. It has been suggested that most colonies die through eventual break up by wave action (Jackson and Hiscock, 2008[68]). Increased exposure will result in a potentially shorter colony life. S. alveolata is a southern species and is at the northern end of its range in Britain. This species is known to be negatively affected by extremely cold winters. In the cold winter of 1962/1963, S. alveolata suffered severe mortalities along the Welsh and southern English coastlines, where it had previously reached its northern and northeastern range limits (Crisp, 1964Cite error: Closing </ref> missing for <ref> tag). Recent work by Mieszkowska et al. (2006)[70] showed that S. alveolata had recolonized locations close to their northern range limits from where they were lost after the cold winter of 1962/1963. Despite the current trends in global warming, winter 2009/2010 was the coldest on record in Europe, which may have negatively affected S. alveolata at its range edges. Continued monitoring is necessary to detect future changes.
Chemical threats
There is insufficient information available on chemical threats to S. alveolata.
Biological threats
There is very little information available on the biological threats to S. alveolata. In a recent study of S. alveolata reefs in the Bay of Mont San‐Michel, France found that reefs were becoming increasingly colonized by the invasive Pacific oyster Crassostrea gigas from local aquaculture operations and by green algae (Ulva spp.) due to the increasing inputs of nitrates from terrestrial origin (Dubois et al., 2006[71]). It was found that epibionts, especially green algae, alter S. alveolata population structure, causing a reduction in new recruits that in the long run may cause significant damage to the reef structure itself. Furthermore, Dubois et al. (2006)[71] noted that C. gigas have high filtration rates, suggesting that they may out-compete S. alveolata for food.
Competition for space with common mussels Mytilus spp. occurs, especially on boulder scars, but the factors influencing this are unknown. Heavy settlement of mussels on S. alveolata reefs has been suspected of causing short term destabilization and loss of habitat (Tyler -Walters, 2008[64]).
Mytilus spp.
Physical threats
Mytilus spp. can be found both intertidally and subtidally. It is a fairly tolerant species with the biggest threats posed by habitat loss and dislodgement by storms. Removal of the substratum, be it rock or sediment, will entail removal of the entire population and its associated community. Repeated substratum loss and recruitment result in a patchy distribution of mussels on the shore (Seed and Suchanek, 1992[44]). Storms and tidal surges are known to destroy mussel beds, often over hundreds of hectares in the Wash, Morecambe Bay and the Wadden Sea. With increasing wave exposure, mussel beds become increasingly patchy and dynamic. Mytilus spp. beds may also be damaged by wave driven logs or equivalent debris (Seed and Suchanek, 1992Cite error: Closing </ref> missing for <ref> tag). The combined effects of trampling and natural winter disturbances may result in loss of mussel beds in the long term. Displacement and or dislodgement by storms will likely lead to mortality. Dare (1976)[49] found that individual mussels swept or displaced rarely survived, since they either became buried in sand or mud, or were scattered and eaten by oystercatchers.
Chemical threats
In general, Mytilus spp. is tolerant of a wide range of contaminants and salinity and oxygen fluctuations. The most significant natural chemical threat to Mytilus spp. is a reduction in salinity caused by storm runoff (Hiscock pers. Comm. in Tyler-Walters 2008[64]). The effects of contaminants on Mytilus sp. were extensively reviewed by Widdows and Donkin (1992)[72] and Livingstone and Pipe (1992)[73]. Mussels are suspension feeders and therefore process large volumes of water together with suspended particulates and phytoplankton. Mussels absorb contaminants directly from the water, through their diet and via suspended particulate matter (Widdows and Donkin, 1992)[72], the exact pathway is dependant on the nature of the contaminant.
Biological threats
Mytilus spp. host a wide variety of disease organisms, parasites and commensals from many animal and plant groups including bacteria, blue green algae, green algae, protozoa, boring sponges, boring polychaetes, boring lichen, the intermediary life stages of several trematodes, copepods and decapods (Bower, 1992[74]; Gray et al., 1999[75]). Mytilus spp. is threatened by a number of invasive species. Aulocomya ater, a mytilid, native to South America has been reported in the Moray Firth, Scotland in 1994 and again in 1997 (Holt et al., 1998[41]; Eno et al., 2000; McKay, 1994[76]). A. Ater is thought to have a stronger byssal attachment than Mytilus spp. and can replace Mytilus spp. in more exposed areas if it reproduces successfully (Holt et al., 1998[41]).
The Pacific oyster Crassostrea gigas was introduced in Europe for commercial purposes in the mid 1960s. In Europe, wild populations of Pacific oysters are already found from northern Germany to southern Portugal. Fey et al. (2010)[77] found that many mussel beds (Mytilus spp.) have been taken over by Pacific oysters in the Dutch Wadden Sea. In the German Wadden Sea almost all mussel beds are now considered oyster reefs (Nehls et al., 2006[78]; Wehrmann et al., 2007[79]). In the early stage of the development of C. gigas, Reise (1998) found 85% attached to Mytilus spp. (alive and empty shell) and 8% on other bivalves.
The American slipper limpet Crepidula fornicata, native to the North American East coast, was unintentionally introduced to Europe by oyster farming in the 1870s and now occurs from Denmark to Spain, Norway, the Mediterranean, Ireland and the United Kingdom (Blanchard, 1997[80]; Thieltges et al., 2003[81]; Rayment, 2007). There are conflicting results in the literature on the effects of C. fornicate on Mytilus spp.. In one set of field experiments (Thieltges, 2005[82]), the presence of C. fornicate has been shown to cause a reduction in survival and growth of the blue mussel Mytilus spp.. A reduction in survival and growth of mussels was likely due to physical interference, associated with the attachment of C. fornicata. It is probable that when attachment onto a host occurs, the host organism will experience greater drag forces, requiring them to use more energy to remain attached to the substrate. This extra energetic requirement may result in reduced fecundity and survivability. Conversely, C. fornicate have also been found to benefit Mytilus spp. Work done by the same authors, Thieltges (2005[82]) found that C. fornicate presence on mussels led to a three‐fold decrease in predation by starfish. Although starfish did not prey directly on C. fornicate, it is believed that the cover provided by settled limpets made it more difficult for the starfish to prey on the mussels.
Modiolus modiolus
Physical threats
M. modiolus is thought to have an intermediate to high intolerance to physical disturbance (Tyler-Walters, 2008[64] and 2007[65] respectively). Subtidal M. modiolus beds are susceptible to damage from fishing activities. In Strangford Lough, Northern Ireland, M. modiolus beds have been shown to suffer damage and mortality by scallop dredging (Service and Magorrian, 1997[83]; Magorrian and Service, 1998[84]).
Chemical threats
There is insufficient information available on chemical threats to M. modiolus.
Biological threats
Predation by crabs and starfish presents one of the greatest threats to juvenile M. modiolus (Brown and Seed, 1977[85]; Anwar et al., 1990[57]; Tyler-Walters, 2007[65]). As mussels grow and become more difficult to open, the threat of predation becomes less important (Seed and Brown, 1977[58]). High densities of the brittle star, Ophiothrix fragilis, are considered to be capable of having a detrimental effect on M. modiolus beds not only through removal of both food and mussel larvae from the water column (George and Warwick, 1985[17]; Holt et al., 1998[41]).
KEY PROCESSES TO FOCUS ON FOR MAINTAINING ECOSYSTEMS INTEGRITY
In this section we discuss the processes to focus on for maintaining ecosystems integrity in terms of reefs in general and will not go into details for each species.
The spatial and temporal distribution of biogenic reefs can vary on vary small scales (i.e. meters and days) (Foster‐Smith, 2000[86]; Foster-Smith and White, 2001) making it difficult to accurately assess their status using point sampling methods. The ephemeral and unpredictable nature of biogenic reefs poses a challenge to effective management. The establishment of designated sites to protect habitats relies on a certain level of stability. Unless conservation effort can be concentrated on reefs of proven stability, site designation for biogenic reefs can prove unsuccessful.
Hendrick et al. (2011)[27] suggest the designation of a much broader site comprising areas which already support dense populations or are considered suitable for potential biogenic reef development may be more beneficial. This approach is analogous to the protection of mobile species rather than habitats or sessile species, affording protection of the environmental condition and mechanisms which enable biogenic reefs to develop. An alternative approach, suggested by Hendrick et al. (2011)[27], is the smaller-scale conservation of specific reef sites, with the view to the designation status lasting only for the lifetime of the actual reef. In order for this approach to be effective, the designation procedure must act on a shorter time scale (months rather than years).
Ideally, a combination of the two above mentioned approaches would prove to be the most effective. This would involve regular mapping of biogenic reefs within a larger supporting boundary. Exclusion zones around the reefs could be established and managed.
CURRENT MANAGEMENT PRACTICES
Biodiversity is of immense interest for managers and policy-makers. As such, The United Nations declared 2010 the International Year of Biodiversity (Resolution 61/203). Throughout the course of the year events will take place world-wide to raise public awareness of not only the biological diversity on our planet, but the importance of protecting it. The origins of legal mechanisms and targets for protecting biodiversity mostly stem from the Convention on Biological Diversity (CBD) that was drawn up in 1992. Parallel to the CBD, the European Community (EC) adopted the Council Directive 92/43/EEC in 1992, this legalization became more commonly known as the Habitats Directive. The directive focused on the conservation of natural habitats and of wild fauna and flora through the establishment of a network of Special Areas of Conservation (SACs). The primary objective of which, is to promote the safeguarding and preservation of threatened species and habitats deemed to be of European importance.
In response to the CBD, the UK Government also founded the UK Biodiversity Partnership to develop and implement UK Biodiversity Action Plans (UK BAP). UK BAP recognizes threatened biological assets within the UK and its surrounding waters and presents policies for the management and conservation of these assets. Plans for species and habitats in danger have been established to aid in recovery in order to assist in the UK’s development in reducing biodiversity loss set out in the CBD (UK Biodiversity Group, 1999). To date, it has lead to the construction of action plans for 1150 priority species and 65 priority habitats (BRIG, 2007]). Reefs are one of the habitats listed under Annex I of the Habitats Directive which require the designation of an SAC.
Sabellaria spinulosa
Intertidal protection for S. alveolata reefs can be achieved through SSSI designation. S. alveolata reefs also occur as sub-features of non‐reef Annex 1 habitats (eg intertidal mudflats and sandflats) under the Habitats Directive and are present in a number of candidate Special Areas of Conservation (cSACs). Discharges to the sea are controlled by a number of EC Directives, including the Dangerous Substances, Shellfish (Waters), Integrated Pollution Control, Urban Waste Water Treatment, and Bathing Waters Directives. The forthcoming Water Framework Directive will also be relevant. The Oslo and Paris Convention (OSPAR) and North Sea Conference declarations are also important. These commitments provide powers to regulate discharges to the sea and have set targets and quality standards to marine waters. An extensive set of standards covering many metals, pesticides and other toxic, persistent and bioaccumulative substances, and nutrients have been set under UK legislation.
Sabellaria alveolata
Intertidal protection for S. alveolata reefs can be achieved through SSSI designation. S. alveolata reefs also occur as sub‐features of non‐reef Annex 1 habitats (eg intertidal mudflats and sandflats) under the Habitats Directive and are present in a number of candidate Special Areas of Conservation (cSACs). Discharges to the sea are controlled by a number of EC Directives, including the Dangerous Substances, Shellfish (Waters), Integrated Pollution Control, Urban Waste Water Treatment, and Bathing Waters Directives. The forthcoming Water Framework Directive will also be relevant. The Oslo and Paris Convention (OSPAR) and North Sea Conference declarations are also important. These commitments provide powers to regulate discharges to the sea and have set targets and quality standards to marine waters. An extensive set of standards covering many metals, pesticides and other toxic, persistent and bioaccumulative substances, and nutrients have been set under UK legislation.
Mytilus spp.
Alhough Mytilus spp. is not designated under any protection laws, the habitat “Intertidal Mytilus spp. beds on mixed and sandy sediments” has been listed on the OSPAR List of Threatened and/or Declining Species and Habitats. Mytilus spp. is also protected by fisheries regulations. Fisheries regulations vary greatly in different parts of the Europe. The regulatory considerations in terms of mussel fisheries management are complex.
Modiolus modiolus
In addition to its listing by OSPAR, this habitat is the subject of several local, national and regional listings, including the Habitats Directive (as part of ‘Reefs’) and the UK Biodiversity Action Plan. Such listings serve to highlight the conservation needs of the habitat, but successful protection depends on specific actions that follow. In the UK M. modiolus beds are identified as features for protection in SACs (Special Areas of Conservation) off Scotland, Wales and Northern Ireland.
SEE ALSO
Natural barriers, Biogenic reefs
Biogenic reefs of Europe and temporal variability
REFERENCES
- ↑ 1.0 1.1 1.2 Biogenic reefs of Europe and temporal variability
- ↑ Natural barriers
- ↑ JACKSON J., 1977. Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. The American Naturalist. 111, 743-767.
- ↑ WOOD R., 1999. Reef Evolution. Oxford University Press, Oxford. pp. 414. Availbale from: [1]
- ↑ CHISHOLM J.R.M., & KELLEY R., 2001. Worms start the reef-building process. Nature. 409, 152 153.
- ↑ 6.0 6.1 CONNOR D., DALKIN M., HILL T., HOLT R. & SANDERSON W., 1997. Marine Nature Conservation Review: marine biotope classification for Britain and Ireland. Volume 2. Sublittoral biotopes. Version 97.06. Joint Nature Conservation Committee, Peterborough. pp 448. Available from: [2]
- ↑ HOLT T., HARTNOLL R. & HAWKINS S., 1997. Sensitivity and vulnerability to man‐induced change of selected communities: intertidal brown algal shrubs, Zostera beds and Sabellaria spinulosa reefs. English Nature Research Reports. No. 234. pp97.
- ↑ WARREN P.J., SHELDON R.W., 1967. Feeding and migration patterns of the Pink Shrimp Pandalus montagui, in the estuary of the River Crouch, England, Journal of the Fisheries Research Board of Canada. 24, 569-580.
- ↑ 9.0 9.1 SCHAFER W., 1972. Ecology and Palaeoecology of Marine Environments. Translation of Aktuo-paläontologie nach Studien in der Nordsee. University of Chicago Press, Chicago. pp 568. Availbale from: [3]
- ↑ WARREN P., 1973. The fishery for the pink shrimp Pandalus montagui of the Wash. Laboratory Leaflet (New Series) No. 28. Ministry of Agriculture, Fisheries and Food, Lowestoft. pp. 46.
- ↑ LIMPENNY D.S., FOSTER‐SMITH R.L., EDWARDS T.M., HENDRICK V.J., DIESING M., EGGLETON J.D., MEADOWS W.J., CRUTCHFIELD Z., PFEIFER S., & REACH I.S., 2010.Best methods for identifying and evaluating Sabellaria spinulosa and cobble reef. Aggregate Levy Sustainability Fund Project MAL0008. Joint Nature Conservation Committee, Peterborough. pp 134.
- ↑ KIRTLEY D.J., 1992. Built to last. Worm reefs. A feat of natural engineering. Florida Oceanographic Magazine. 13, 12‐19.
- ↑ JONES L., 1999. Habitat Action Plan: Sabellaria spinulosa reefs. English Nature. pp 4.
- ↑ JONES L.A., HISCOCK K., CONNOR D.W., 2000. Marine habitat reviews. A summary of ecological requirements and sensitivity characteristics for the conservation and management of marine SACs. Joint Nature Conservation Committee, Peterborough. (UK Marine SACs Project report).
- ↑ FOSTER‐SMITH R.L., 2001. Report of the field survey for the 2001 Sabellaria spinulosa project. A report for the Eastern Sea Fisheries Joint Committee and English Nature. pp 45.
- ↑ CASPERS H., 1950. Die Lebensgemeinschaft der Helgolander Austernbank. Helgoland Marine Research. 3, 119-169. Available from: [4].
- ↑ 17.0 17.1 17.2 17.3 GEORGE C., & WARWICK R., 1985. Annual production in a hard‐bottom reef community. Journal of the Marine Biological Association of the United Kingdom. 65, 713-735. Availble from: [5].
- ↑ 18.0 18.1 JESSOP R. & STOUTT J., 2006. Broad scale Sabellaria spinulosa distribution in the central Wash (Southern North Sea), as predicted with the acoustic ground discriminating system (A.G.D.S) RoxannTM. Draft report by the Eastern Sea Fisheries Joint Committee for English Nature. pp 26.
- ↑ 19.0 19.1 LINKE O., 1951. Neue Beobachtungen uber Sandkorallen‐Riffe in der Nordsee, Natur u.Volk.. 81, 77-84.
- ↑ 20.0 20.1 20.2 20.3 WILSON D.P., 1971. Sabellaria colonies At Duckpool, North Cornwall, 1961‐1970. Journal of the Marine Biological Association of the UK, 51: 509‐580. Available form: [6]
- ↑ MICHAELIS H., 1978. Recent biological phenomena in the German Waddensea. Symposium on North Sea fish stocks-recent changes and their causes., Aarhus (Denmark).
- ↑ 22.0 22.1 22.2 ECKELBARGER K.J., 1978. Metamorphosis and settlement in the Sabellariidae. In: Chai, F.-S. & Rice, M. (Eds.). Settlement and Metamorphosis of Marine Invertebrate Larvae.Proceedings of the Symposium on Settlement and Metamorphosis of Marine Invertebrate Larvae, American Zoological Society Meeting. Totonto, Ontario, Canada December 27-28, 1977. Elsevier, New York: pp. 145-164.
- ↑ WILSON D.P., 1929. The larvae of the British Sabellarians. Journal of the Marine Biological Association of the United Kingdom. 15, 221‐269.
- ↑ 24.0 24.1 24.2 24.3 WILSON D.P., 1968.The settlement behavior of the larvae of Sabellaria alveolata. Journal of the Marine Biological Association of the United Kingdom. 48, 387‐435.
- ↑ WILSON D.P., 1970. The larvae of Sabellaria Spinulosa and their settlement behaviour. Journal of the Marine Biological Association of the United Kingdom. 50, 33-52. Available from: [7]
- ↑ JENSEN R.A., 1992. Marine bioadhesive: role for chemosensory recognition in a marine invertebrate. Biofouling. 5, 177-193.
- ↑ 27.0 27.1 27.2 HENDRICK V. J., FOSTER‐SMITH R. L. & DAVIES A. J., 2011. Biogenic Reefs and the Marine Aggregate Industry. Marine ALSF Science Monograph Series No. 3. MEPF 10/P149. (Edited by R. C. NEWELL & J. MEASURES). 60pp. ISBN: 978 0 907545 46 0.
- ↑ DAVIES A.J., LAST K.S., ATTARD K., HENDRICK V.J., 2009. Maintaining turbidity and current flow in laboratory aquarium studies, a case study using Sabellaria spinulosa. Journal of Experimental Marine Biology and Ecology. 370, 35-40.
- ↑ 29.0 29.1 VORBERG R., 2000. Effects of the shrimp fisheries on reefs of Sabellaria spinulosa (Polychaeta). ICES Journal of Marine Science. 57, 1416-1420.
- ↑ STEWART R.J., WEAVER J.C., MORSE D.E. & WAITE J.H., 2004. The tube cement of Phragmatopoma californica: a solid foam. Journal of Experimental Biology. 207, 4727-4734.
- ↑ BRAITHWAITE C.J.R., ROBINSON R.J., & JONES G., 2006. Sabellarids: a hidden danger or an aid to subsea pipelines? Quarterly Journal of Engineering Geology and Hydrogeology. 39, 259‐265.
- ↑ LAST K.S., HENDRICK V.J., BEVERIDGE C.M. & DAVIES A.J., 2011. Measuring the effects of suspended particulate matter and smothering on the behaviour, growth and survival of key species found in areas associated with aggregate dredging. Report for the Marine Aggregate Levy Sustainability Fund, Project MEPF 08/P76. 69 pp.
- ↑ KIRTLEY D.J., 1966. Intertidal reefs of Sabellariidae (Annelida polychaeta) along the coasts of Florida. Masters thesis. The Florida State University. Tallahassee, Florida. 104 pp. Original reference not seen. Cited by Drake, C.A., McCarthy, D.A. & von Dohlen, C.D. (2007). Molecular relationships and species divergence among Phragmatopoma spp. (Polychaeta: Sabellaridae) in the Americas. Marine Biology. 150(3), 345‐358.
- ↑ MCCARTHY D., 2001. Life-history patterns and the role of disturbance in intertidal and subtidal populations of the polychaete Phragmatopoma lapidosa lapidosa (Kinberg, 1867) in the tropical Western Atlantic. PhD Thesis. Kings College, University of London. Original reference not seen. Cited by Drake, C.A., McCarthy, D.A. & von Dohlen, C.D. (2007).Molecular relationships and species divergence among Phragmatopoma spp. (Polychaeta: Sabellaridae) in the Americas. Marine Biology. 150(3), 345‐ 358.
- ↑ MCCARTHY D., YOUNG C. & EMSON R., 2003. Influence of wave induced disturbance on seasonal spawning patterns in the sabellariid polychaete Phragmatopoma lapidosa. Marine Ecological Progress Series. 256, 123-133.
- ↑ WILSON D.P., 1974. Sabellaria Colonies at Duckpool, North Cornwall, 1971–1972, With a Note for May 1973. Journal of the Marine Biological Association of the United Kingdom. 54, 393‐436.
- ↑ 37.0 37.1 37.2 37.3 37.4 CUNNINGHAM P.N., HAWKINS S.J., JONES H.D., BURROWS M.T., 1984. The geographical distribution of Sabellaria alveolata (L.). In: England, Wales and Scotland, with investigations into the community structure of, and the effects of trampling on Sabellaria alveolata colonies. Report to the Nature Conservancy Council from the Department of Zoology, Manchester University, Manchester. NCC report No. HF3/11/22.
- ↑ LARSONNEUR C. 1994. The Bay of Mont‐Saint‐Michel: A sedimentation model in a temperate macrotidal environment. Senckenbergiana maritima. 24, 3‐63.
- ↑ WILSON D.P., 1976. Sabellaria Alveolata (L.) At Duckpool, North Cornwall, 1975. Journal of the Marine Biological Association of the United Kingdom. 56, 305-310.
- ↑ 40.0 40.1 GRUET Y., 1982. Recherches sur l’écologie des récifs d’Hermelles édicés par l’Annélide Polychète Sabellaria alveolata (Linné), Université des Sciences et Techniques, Nantes, France. PhD.
- ↑ 41.00 41.01 41.02 41.03 41.04 41.05 41.06 41.07 41.08 41.09 41.10 HOLT T.J., REES E.I., HAWKINS, S.J., SEED, R., 1998. Biogenic Reefs (volume IX). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scottish Association for Marine Science (UK Marine SACs Project). 170 pp.
- ↑ 42.0 42.1 SEED R., 1969. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. Oecologia. 3, 317‐350.
- ↑ ROBERTS D., & MCKENZIE J.D., 1983. Utilisation of mollusk resources in N. Ireland. Journal of Molluscan Studies. 49, 162-166.
- ↑ 44.0 44.1 44.2 44.3 44.4 SEED R. & SUCHANEK T.H., 1992. Population and community ecology of Mytilus. In: Gosling, E. (Ed.). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science. 25, Elsevier, Amsterdam: pp. 87-170. Available from: [8]
- ↑ WIDDOWS J., & SHICK J.M., 1985. Physiological responses of Mytilus edulis and Cardium edule to aerial exposure. Marine Biology. 85, 217-232.
- ↑ 46.0 46.1 MCGRATH D., KING P., & GOSLING E., 1988. Evidence for the direct settlement of Mytilus edulis larvae on adult mussel beds. Marine Ecological Progress Series. 47, 103‐106.
- ↑ Pulfrich, A., 1996; Attachment and settlement of post-larval mussels (Mytilus edulis L) in the Schleswig-Holstein Wadden Sea Source. JOURNAL OF SEA RESEARCH. 36(3-4), 239-250. DOI: 10.1016/S1385-1101(96)90793-5.
- ↑ Beukema, J.J. (1981). Quantitative data on the benthos of the Wadden Sea proper. In: Dankers, N.M.J.A. et al. (1981).Invertebrates of the Wadden Sea: final report of the section 'Marine Zoology' of the Wadden Sea Working Group. Wadden Sea Working Group Report, 4: pp. 134-142. Available from: [9].
- ↑ 49.0 49.1 DARE P.J., 1976. Settlement, growth and production of the mussel, Mytilus edulis L., in Morecambe Bay, England. Fishery Investigations, Ministry of Agriculture, Fisheries and Food. Pp 25. Original reference not seen. Cited by Tyler‐Walters, H. (2008). Mytilus edulis. Common mussel. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom.
- ↑ SEED R., 1976. Ecology. In: Bayne, B. (Ed.). Marine mussels: their ecology and physiology. International Biological Programme 10. Cambridge University Press, Cambridge: pp. 13‐66. Available from: [10].
- ↑ SUKHOTIN A.A., STRELKOV P.P., MAXIMOVICH N.V. & HUMMEL H., 2007. Growth and longevity of Mytilus edulis (L.) from northeast Europe. Marine Biology Research. 3, 155-167. Available from: [11].
- ↑ HOLT T.J., & SHALLA S.H.A., 1997. Pre- and post-drilling survey of block IOM 112/19, A report to Elf Enterprise Caledonia Ltd. By Port Erin Marine Laboratory, University of Liverpool. Unpublished work.
- ↑ SCHWEINITZ E., & LUTZ R., 1976. Larval development of the northern horse mussel, Modiolus modiolus (L.), including a comparison with the larvae of Mytilus edulis L. as an aid in planktonic identification. Biological Bulletin. 150, 348‐360.
- ↑ 54.0 54.1 DAVENPORT J. & KJORSVIK E., 1982. Observations on a Norwegian intertidal population of the horse mussel Modiolus modiolus (L.). Journal of Molluscan Studies. 48, 370‐371.
- ↑ COLEMAN N.,1976. Aerial respiration of Modiolus modiolus. Comparative Biochemistry and Physiology Part A: Physiology. 54, 401‐406.
- ↑ WILDISH D.J., FADER G. & PARROTT D., 2009. A model of horse mussel reef formation in the Bay of Fundy based on population growth and geological processes. Atlantic Geology. 45, 157-170.
- ↑ 57.0 57.1 ANWAR N. A., RICHARDSON C.A., & SEED R., 1990. Age determination, growth rate and population structure of the horse mussel Modiolus modiolus. Journal of the Marine Biological Association of the United Kingdom. 70, 441-457.
- ↑ 58.0 58.1 SEED R., & BROWN R.A., 1977. Comparison of reproductive cycles of Modiolus modiolu (L), Cerastoderma (= Cardium) edule (L), and Mytilus edulis L in Strangford Lough, Northern Ireland. Oecologia. 30, 173-188. Available from: [12].
- ↑ 59.0 59.1 BROWN R.A. 1984. Geographical variations in the reproduction of the horse mussel, Modiolus modiolus (Mollusca: bivalvia). Journal of the Marine Biological Association of the United Kingdom. 64, 751-770.
- ↑ JASIM A.K., & BRAND A.R., 1989. Observations on the reproduction of Modiolus modiolus in Isle of Man waters. Journal of the Marine Biological Association of the UK. 69, 373-385.
- ↑ 61.0 61.1 61.2 61.3 61.4 61.5 61.6 www.marlin.ac.uk
- ↑ Lewis, 1977: The role of physical and biological factors in the distribution and stability of rocky shore communities Lewis, J.R. (1977). The role of physical and biological factors in the distribution and stability of rocky shore communities. In: Keegan, B.F. et al. (Ed.) (1977). Biology of Benthic Organisms: 11th European Symposium on Marine Biology, Galway, 1976. pp. 417-424.
- ↑ LUTZ R.A., & KENNISH M.J., 1992. Ecology and morphology of larval and early postlarval mussels. In: Gosling, E. (Ed.). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science 25. Elsevier Press, Amsterdam: pp. 53‐86. Available from: [13]
- ↑ 64.0 64.1 64.2 64.3 TYLER-WALTERS H., 2008. Mytilus edulis. Common mussel. Marine Life Information Network: Biology and Sensitivity Key Information Sub-programme [on‐line]. Plymouth: Marine Biological Association of the United Kingdom. More info: [14].
- ↑ 65.0 65.1 65.2 TYLER-WALTERS H., 2007. Modiolus modiolus. Horse mussel. Marine Life Information Network: Biology and Sensitivity Key Information Sub‐programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom. [cited 01/05/2011]. more info: [15].
- ↑ COMELY C.A. 1978. Modiolus modiolus (L.) from the Scottish west coast. Ophelia. 17, 167-193.
- ↑ CONNOR D.W., ALLEN J.H., GOLDING N., HOWELL K.L. LIEBERKNECHT L.M., NORTHEN K.O. & REKER J.B., 2004. The Marine Habitat Classification for Britain and Ireland. Version 04.05 (internet version: [16]). Joint Nature Conservation Committee, Peterborough. Also available from: [17]
- ↑ 68.0 68.1 68.2 68.3 JACKSON A. & HISCOCK K., 2008. Sabellaria spinulosa. Ross worm. Marine Life Information Network: Biology and Sensitivity Key Information Sub‐programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom. Available from: [18].
- ↑ CRISP D.J. 1964. The effects of the severe winter of 1962‐63 on marine life in Britain. Journal of Animal Ecology. 33, 165‐210.
- ↑ MIESZKOWSKA N., KENDALL M.A., HAWKINS S.J., Leaper R., Williamson P., Hardman-Mountford N.J., SOUTHWARD A.J., 2006. Changes in the range of some common rocky shore species in Britain - a response to climate change? Hydrobiologia. 555, 241‐51. Available from: [19].
- ↑ 71.0 71.1 DUBOIS S., COMMITO J.A., OLIVIER F., & RETIERE C., 2006. Effects of epibionts on Sabellaria alveolata (L.) biogenic reefs and their associated fauna in the Bay of Mont Saint-Michel. Estuarine, Coastal and Shelf Science. 68, 635‐646.
- ↑ 72.0 72.1 WIDDOWS J., & DONKIN P., 1992. Mussels and environmental contaminants: bioaccumulation and physiological aspects. In: Gosling, E. (Ed.). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science 25. Elsevier Press, Amsterdam: pp.383‐424.
- ↑ LIVINGSTONE D.R., & PIPE R.K., 1992. Mussels and environmental contaminants: molecular and cellular aspects. In: Gosling, E. (Ed.). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science 25. Elsevier Press, Amsterdam: pp. 425-510.
- ↑ BOWER S.M., 1992. Diseases and parasites of mussels. In: Gosling, E. (Ed.). The mussel Mytilus: ecology, physiology, genetics and culture. Developments in Aquaculture and Fisheries Science 25. Elsevier, Amsterdam: pp. 543‐563. Available from: [20].
- ↑ GRAY A.P., LUCAS I.A.N., SEED R., and RICHARDSON C.A., 1999 Mytilus edulis chilensis infested with Coccomyxa parasitica (Chlorococcales, Coccomyxaceae). Journal of Molluscan Studies. 65, 289-294.
- ↑ MCKAY D., 1994. Unravelling the choreography of contaminant kinetics: approaches to quantifying the uptake of chemicals by organisms. In: J.L. Hamelink, P.F. Landrum, H.L. Bergman and W.H. Benson (Editors), Bioavailability: Physical, Chemical, and Biological Interactions, Lewis Publisher Inc., Chelsea, MI., pp. 17l‐l77.
- ↑ FEY F., DANKER N., STEENBERGEN J., & GOUDSWAARD K., 2010. Development and distribution of the non-indigenous Pacific oyster (Crassostrea gigas) in the Dutch Wadden Sea. Aquaculture International. 18(1), 45‐59. Available form: [21].
- ↑ NEHLS G., DIEDERICH S., THIELTGES D., & STRASSER M., 2006. Wadden Sea mussel beds invaded by oysters and slipper limpets: competition or climate control? Helgoland Marine Research. 60, 135‐143. Available from: [22].
- ↑ WEHRMANN A, MARKERT A, SCHMIDT A., 2007 Miesmuschelbank: ein verlorener Lebensraum? Die Einwanderung der Pazifischen Auster in das Wattenmeer und ihre Folgen. Natur- und Umweltschutz. 6(1), 10–14.
- ↑ BLANCHARD M., 1997. Spread of the slipper‐limpet (Crepidula fornicata) in Europe. Current state and consequences. Scientia Marina.61(2 sup.), 109-118. Available from: [23].
- ↑ THIELTGES D.W., STRASSER M., REISE K., 2003. The American slipper limpet Crepidula fornicate (L.) in the northern Wadden Sea 70 years after its introduction. Helgoland Marine Research. 57, 27-33.
- ↑ 82.0 82.1 THIELTGES D.W., 2005a. Impact of an invader: epizootic American slipper limpet Crepidula fornicate reduces survival and growth in European mussels. Marine Ecology Progress Series. 286,13-19.AND THIELTGES D.W., 2005b. Benefit from an invader: American slipper limpet Crepidula fornicate reduces star fish predation on basibiont European mussels. Hydrobiologia. 541(1), 241‐244. Available from: [24]
- ↑ SERVICE M., MAGORRIAN B. H., 1997. The extent and temporal variation of disturbance of epibenthic communities in Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom. 77, 1151‐1164.
- ↑ MAGORRIAN B.H., & Service, M., 1998. Analysis of underwater visual data to identify the impact of physical disturbance on horse mussel (Modiolus modiolus) beds. Marine Pollution Bulletin. 36, 354-359.
- ↑ BROWN R.A. & SEED R., 1977. Modiolus modiolus (L.) - an autecological study. In: KEEGAN B.F., O'CEIDIGH P., BOADEN P.J.S. (eds). Biology of Benthic Organisms. Proceedings of the 11th European Symposium on Marine Biology, Pergamon Press, Oxford, Galway, Ireland, pp 93‐100. Available from: [25].
- ↑ FOSTER‐SMITH R.L., 2000. Establishing a monitoring baseline for the Wash subtidal sandbanks. pp 51.
Please note that others may also have edited the contents of this article.
|
