Difference between revisions of "Mangroves"
Dronkers J (talk | contribs) |
|||
| (75 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | This article describes the habitat of the | + | |
| + | This article describes the [[habitat]] of the mangrove forests. It is one of the subcategories within the section dealing with the biodiversity of [[marine habitats and ecosystems]]. It provides an overview of the characteristics, distribution, biota, functioning and adaptation to habitat conditions. An introduction is given to management aspects, discussing threats, conservation and [[Ecosystem rehabilitation|rehabilitation]] of mangrove forests. | ||
==Introduction== | ==Introduction== | ||
| − | Mangroves are the only trees that are capable of thriving in salt water. They form unique intertidal forests at the edge of land and sea. They are represented on all continents with tropical and subtropical coasts, i.e. North and South America, Africa and Middle-East, Asia and Oceania (incl. Australia). <ref>http://www.vliz.be/vmdcdata/mangroves</ref> | + | Mangroves are the only trees that are capable of thriving in salt water. They form unique [[intertidal]] forests at the edge of land and sea, see Fig. 1. They are represented on all continents with tropical and subtropical coasts, i.e. North and South America, Africa and Middle-East, Asia and Oceania (incl. Australia). <ref name="vliz">http://www.vliz.be/vmdcdata/mangroves</ref> |
| + | |||
| + | Mangrove forests or '''mangals''' are a type of [[intertidal]] wetland [[ecosystems]]. The word mangrove is derived from the Portugese word mangue which means “tree” and the English word grove which is used for trees and shrubs that are found in shallow, sandy or [[mud]]dy areas (Karleskint, 1998<ref name=K98>Karleskint G. 1998. Introduction to marine biology. Harcourt Brace College Publishers. p.378</ref>). They replace [[salt marsh]]es in tropical and subtropical regions. | ||
| + | They are salt-tolerant forested [[wetlands]] at the interface between the terrestrial landscape and the marine environment. The dominant vegetation are several species of mangrove: woody trees and shrubs with a thick, partially exposed network of roots that grow down from the branches into the water and sediment. They settle where there is little [[waves|wave action]] and where muddy [[sediments]] accumulate. While growing, mangal forests further reduce waves and increase sedimentation. Wave energy reduction can be greater than 50% on average and increases with increasing offshore wave heights (Horstman et al., 2014<ref name=H14>Horstman, E.M., Dohmen-Janssen, C.M., Narra, P.M.F., van den Berg, N.J.F., Siemerink, M. and Hulscher, S.J.M.H. 2014. Wave attenuation in mangroves: A quantitative approach to field observations. Coastal Engineering 94: 47–62</ref>). Mangals therefore fulfill an important coastal protection function. | ||
| + | Mangroves are frequently associated with saline [[Lagoon|lagoons]] and are regularly found on protected sides of islands, [[Island atolls|atolls]] and tropical [[estuaries]] (Karleskint, 1998<ref name=K98/>). | ||
| + | |||
| + | |||
| + | [[image:mangrove thailand.jpg|center|thumb|450px|caption|Fig. 1. Mangal in Thailand.]] | ||
| − | + | ==Requirements for development== | |
| − | |||
| − | |||
| − | |||
| + | Requirements for the development of mangroves are: | ||
| + | * Average temperature of the coldest month higher than 20°C; the seasonal temperature range should not exceed 5°C. They are not resistant to freezing. | ||
| + | * A fine-grained substrate. Exceptions are the development of mangroves on [[Coral reefs|corals]], as for example in Papua New Guinea and Kenya. | ||
| + | * Shores must be free of strong [[waves|wave]] action and strong [[tidal current]]s. | ||
| + | * Saline water; they are facultative halophytes. | ||
| + | * Deposition of sediments by small-moderate waves and tides. | ||
| + | {| {| style= border="0" align="right" | ||
| + | |- | ||
| + | | valign="top"| | ||
| + | [[File:RedMangrove.jpg|thumb|200px|caption|Red mangrove (''Rhizophora'')]] | ||
| + | | valign="top"| | ||
| + | [[File:BlackMangrove.jpg|thumb|250px|caption|Black mangrove (''Avicennia'')]] | ||
| + | |} | ||
| − | |||
| + | Due to these requirements, a well-marked [[zonation]] is seen. Each of the zones is dominated by a different mangrove species and associated fauna and flora. Red mangroves (''Rhizophora'') are usually found closest to the edge of the water, where the greatest degree of tidal flooding occurs. More landwards are the black mangroves (''Avicennia''). These areas receive only shallow flooding during high tide. The upper limit of the mangroves is occupied with white mangroves and buttonwoods. The buttonwoods are not really a mangrove species, but are a transition species between the mangrove and the terrestrial vegetation. | ||
==Distribution== | ==Distribution== | ||
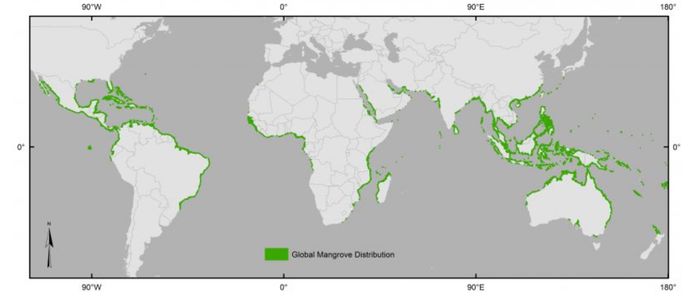
| − | The distribution, density and species composition are determined by the water and air temperatures during the winter, exposure to wave action and [[ | + | The area covered by mangroves worldwide is estimated at almost 150,000 km<sup>2</sup> <ref>Bunting, P., Rosenqvist, A., Hilarides, L., Lucas, R.M., Thomas, T., Tadono, T., Worthington, T.A., Spalding, M., Murray, N.J. and Rebelo, L-M. 2022. Global Mangrove Extent Change 1996 – 2020: Global Mangrove Watch Version 3.0. Remote Sensing. https://zenodo.org/records/6894273</ref>. The distribution, density and species composition are determined by the water and air temperatures during the winter, exposure to [[waves|wave action]] and [[tidal current]]s, the range of the [[tide]], the type of [[sediment]] and the chemistry of the seawater. The global distribution of mangroves is shown in Fig. 2. |
| − | The most highly developed and most species rich mangals are found in Malaysia | + | The most highly developed and most species-rich mangals are found in Indonesia, Australia and Malaysia. Over the world, 54-70 species and hybrids in 20-27 genera and 16-19 families are found (Berness et al., 2001<ref>Bertness M.D., Gaines, S.D. and Hay, M.E. 2001. Marine Community Ecology. Sinauer Associates, Inc. p. 550</ref>). A species overview is given in the Mangrove Species Database <ref name="vliz"/>. |
| + | |||
| + | |||
| + | Mangroves are almost exclusively tropical, but also occur in the subtropics. They do not tolerate frost, but can cope with air temperatures down to 5 °C. Their occurrence is most closely related to seawater temperature. The isotherm of 20 °C in winter is a good indicator of the distribution limit. ''Avicennia'' appears to be more tolerant of low temperatures than ''Rhizophora'' or ''Laguncularia''. The [[Species diversity | number of species]] tends to decrease with distance from the equator. In the Southern Hemisphere, mangals generally occur further south on the eastern edges of landmasses than on the western side. This is due to the pattern of hot and cold [[Ocean circulation | ocean currents]] (Pinet, 1998<ref>Pinet P.R. 1998.Invitation to Oceanography. Jones and Barlett Publishers. p. 508</ref>; Hogarth, 1999 <ref name=H99/>). | ||
| + | |||
| + | |||
| + | [[image:MangroveWorldMap.jpg|center|thumb|700px|caption|Fig. 2. World distribution of mangroves.]] | ||
| + | |||
| + | |||
| + | As a result of global warming, the area with a suitable settling climate for mangroves is currently expanding in the polar direction. Colonization of salt marshes has been observed along several continents in recent decades, especially by the most cold-tolerant mangrove genus ''Avicennia'' (Saintilan et al., 2014<ref>Saintilan, N., Wilson, N., Rogers, K., Rajkaran, A. and Krauss, K.W. 2014. Mangrove expansion and saltmarsh decline at mangrove poleward limits. Glob. Change Biol. 20: 147–57</ref>). | ||
| + | |||
| + | ==Functioning and adaptations== | ||
| − | + | Although mangrove species are unrelated taxonomically, they exhibit similar morphological, physiological and reproductive traits. Mangroves have several functions and adaptations for thriving in saline [[intertidal]] zones. They grow in an environment whose [[salinity]] ranges between freshwater and seawater. For this reason, they have to take up water against the [[Osmosis|osmotic pressure]]. To overcome the negative osmotic pressure, they generate a negative hydrostatic pressure (by transpiration processes). Roots or leaves exude salt, which make them tolerant to saline conditions. Even after most of the salts have been removed, concentration of chloride and sodium ions in the tissue is higher than in other plants. Salt is stored in '''vacuoles''' to protect enzymes that might otherwise be inhibited. The high cation concentrations are balanced by high non-ionic solutes in the cytoplasm. Several mangrove species deposit sodium and chloride in the bark of stems and roots. Other species deposit salt in senescent leaves, which later fall off the tree. '''Salt glands''' on the leaves also exude salt that forms crystals. In some species, fine hairs covering the lower leaf surface raise the secreted droplets of salt water away from the leaf surface in order to prevent osmotic withdrawal of water from the leaf tissues (Parida and Jha, 2010<ref>Parida, A. K. and Jha, B. 2010. Salt tolerance mechanisms in mangroves: A review. Trees 24: 199–217</ref>). | |
| + | Mangroves also need adaptations to conserve water. Leaves have a thick waxy cuticle (skin on the leaf) or dense hairs to reduce transpiration, or orientate their leaves to avoid the burning sun. Most evaporation loss occurs through stomata - pores in the leaves - so these are often sunken below the leaf surface where they are protected from drying winds. Leaves are also commonly succulent, storing water in fleshy internal tissue. | ||
| − | + | Because salt tolerance is costly, a greater relative root mass is needed to recover the demand for water. When it rains, '''drop roots''', roots that descend from the branches, absorb the freshwater that runs down from the stem through a special superficial layer. In this way, no high-energy inverse [[osmosis]] is needed. | |
| − | == | + | {| style= border="0" align="center" |
| + | |- | ||
| + | | valign="top" align=centre| | ||
| + | [[File:Salt crystals.jpg|thumb|120px|Salt crystals on a mangrove leaf <ref name="wikipedia">http://en.wikipedia.org/wiki/Mangrove</ref>]] | ||
| + | | valign="top"| | ||
| + | [[File:Extended root mass.JPG|thumb|250px|Extended root mass. Photo credit Eric Coppejans <ref name="eric"> Eric Coppejans - http://www.vliz.be/imis/imis.php?module=person&persid=134</ref>.]] | ||
| + | | valign="top"| | ||
| + | [[File: Drop roots.jpg|thumb|250px|Drop roots from branches <ref>http://sofia.usgs.gov/virtual_tour/ecosystems/index.html</ref>]] | ||
| + | |} | ||
| − | |||
| + | Deposits of fine grained (muddy and sandy) sediments lack oxygen (Hogarth, 1999<ref name=H99>Hogarth P.J. 1999. The biology of mangroves. Oxford University Press. p.228</ref>). | ||
| + | Mangroves therefore have to cope with anoxic conditions. The tissue of the plants requires oxygen for [[respiration]] which cannot diffuse sufficiently into soils that are waterlogged. Even if the surface water is saturated with oxygen, its concentration in the groundwater is too low. This is why mangroves develop various forms of aerial roots. | ||
| + | * Most of the roots branch off from the stem underground. One type of roots is the '''prop root''' (also called '''stilt root'''). This root diverges from the tree and anchors into the bottom to stabilize the tree in the soft, [[mud]]dy substrate. The rhizophore has '''lenticels''' on the upper surface, large pores with a corky layer enabling the uptake of oxygen. Seawater cannot get into the lenticels. The tissue of the prop roots consists of '''[https://en.wikipedia.org/wiki/Aerenchyma aerenchyma]''' and is connected with the lenticels. Through this aerenchyma, oxygen can be provided to the submerged parts of the tree. The roots that break at alternating places through the soil surface and submerge again form a '''knee root'''. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | {| {| style= border="0" align="center" | ||
| + | |- | ||
| + | | valign="top"| | ||
| + | [[File: Knee Roots detail.JPG|left|thumb|300px|caption|Knee roots. Photo credit Eric Coppejans <ref name="eric"/>]] | ||
| + | | valign="top"| | ||
| + | [[File: 799px-Mangrove.jpg|none|thumb|250px|caption|Prop roots growing from the lower trunk <ref name="wikipedia"/>]] | ||
| + | |} | ||
| − | |||
| + | * Another type of roots is a shallow, horizontal root that radiates outwards. The vertical root is called a '''pneumatophore''' and can be as high as several decimeters. These roots also have lenticels and aerenchyma. They can create a huge network of vertical roots. The horizontal root is called the '''cable root'''. | ||
| + | * The '''buttress root''' (also called '''plank root''') is a root that covers the whole space between the upper part of the root and the bottom. | ||
| − | |||
| − | + | {| style= border="0" align="center" | |
| − | + | |- | |
| − | + | | valign="top" align=centre| | |
| + | [[File: Pneumatophores.jpg|left|thumb|300px|caption|Pneumatophores on cable roots <ref name="wikipedia"/>.]] | ||
| + | | valign="top"| | ||
| + | [[File: Buttress roots.JPG|none|thumb|200px|caption|Buttress root (plank root). Photo credit Eric Coppejans <ref name="eric"/>.]] | ||
| + | | valign="top"| | ||
| + | [[File: Propagules.JPG|center|thumb|150px|caption|Propagules. Photo credit Eric Coppejans <ref name="eric"/>.]] | ||
| + | |} | ||
| − | + | Observations of the mangrove species distribution along the Mekong delta coast showed that pneumatophore species (e.g., ''Avicennia alba'') were preferentially found in accreting sites where soil was often inundated and oxygen-poor mud had accumulated. Stilt root species (e.g., ''Rhizophora apiculata'') occurred mostly in erosion sites where stabilizing support is important. Knee root species (e.g., ''Bruguiera parviflora'') dominated in higher sites with more stable substrate<ref>Nguyen, L.T.M., Hoang, H.T., Choi, E. and Park, P.S. 2023. Distribution of mangroves with different aerial root morphologies at accretion and erosion sites in Ca Mau Province, Vietnam. Estuarine, Coastal and Shelf Science 287, 108324</ref>. ''Avicennia marina'', ''Rhizophora apiculata'', ''Bruguiera gymnorrhiza'' and ''Xylocarpus granatum'' are species with high carbon sequestration capacity. ''A. marina'' and ''R. apiculata'' strive on sand-silt soils in the low-medium intertidal zone, while ''B. gymnorrhiza'' and ''X. granatum'' are often found on silt-clay soils in the high-intertidal zone, especially in estuaries<ref>Syahid, L.N., Sakti, A.D., Ward, R., Rosleine, D., Windupranata, W. and Wikantika, K. 2023. Optimizing the spatial distribution of Southeast Asia mangrove restoration based on zonation, species and carbon projection schemes. Estuarine, Coastal and Shelf Science 293, 108477</ref>. | |
| − | |||
| − | |||
| − | |||
| − | |||
| + | Mangroves aid soil formation by trapping debris. Plank roots and pneumatophores accumulate sediments in protected sites and form mangrove peats; sediment trapping is a function of the volume of aboveground roots (Du et al., 2021<ref>Du, Q., Qin,Z., Ming, S. and Zhang, C. 2021 Differences in the vertical accretion of sediment among mangrove species with different aerial root types. Estuarine, Coastal and Shelf Science 256, 107375</ref>). Root production also contributes to soil elevation, even more than leaf, twig and branch litter, due to slow decomposition (Krauss et al., 2014<ref name=K14>Krauss, K.W., McKee, K.L., Lovelock, C.E., Cahoon, D.R., Saintilan, N., Reef, R. and Chen, L. 2014. How mangrove forests adjust to rising sea level. New Phytologist 202: 19–34</ref>). Filamentous [[algae]] help to stabilize the fine sediments trapped by mangroves. They usually form a green-to-red mass over the substrate. They are also a '''filtering system''' for the land run-off and remove the terrestrial organic matter. They are very important [[habitat]]s for many species of small fish, invertebrates and various epiflora and epifauna as well as larger birds. This is called a '''nursery''' function. The mangrove is a major producer of [[detritus]] that will contribute to offshore [[Biological productivity|productivity]] in some seasons (Coppejans <ref name=E>Eric Coppejans – Course Biodiversity of aquatic food webs: from algae to marine mammals UGent</ref>). | ||
| − | + | Pollination of the trees is done by the wind or by organisms. All mangroves disperse their offspring by water. They produce unusually large propagating structures or propagules. The embryo initiates germination on the seed, still attached on the tree and further develops into a propagule. This phenomenon is known as '''vivipary'''. | |
| + | ==Biota== | ||
| − | + | [https://en.wikipedia.org/wiki/Epiphyte Epiphytic] '''micro-algae''' grow on the aerial roots of the trees and on the sediments. The [[algae]] are green ( Chlorophyta), brown (Phaeophyceae), red (Rhodophyta) and blue-green (Cyanophyta). The dense biomass on the aerial roots causes the water to remain on the pneumatophores; the lenticels are no longer functional and oxygen cannot penetrate into the roots. For this reason, the bark regularly falls off the root. This process is called decertification. Vertical [[zonation]] along a single pneumatophore occurs, but there is also a [[zonation]] from the upper limit of the mangroves to the lower limit. | |
| + | Several invertebrate species are found in the mangrove ecosystem. Macrobenthic species are commonly or occasionally present because they are migrating or because they are living in adjacent environments. Crabs are keystone species in the mangrove ecosystem. This means that the presence of this animal in the community makes it possible for many other species to live there. The crabs go through their larval stages in the water beneath the mangroves. When they are mature, they crawl up on the mangroves and feed on the leaves. They can reach high densities and are crucial in the processing of leaf litter. Their burrowing activity modifies the micro-topography of the bottom and aerates the soil. This decreases the sulphide levels in the soil and positively influences the productivity of the trees. An example of a mangrove crab is the fiddler crab ''Uca lacteal''. | ||
| − | [[ | + | An important '''bivalve''' is the purple oyster ''Lopha frons''. This species encrusts the pneumatophores and prop roots. When the [[tide]] is high, barnacles and mussels compete with the oyster for space on the roots. Periwinkles also occur on the roots and stems, as well as on the shells of sedentary organisms attached on them. Snails are important in the turnover of the organic material. |
| − | + | Other species that occur in the mangroves are tunicates, sponges, ants, hermit crabs, shrimps, fishes,… They are a source of nutrition for higher level predators. Species that cannot tolerate changing saline conditions can survive in the forest. These species are sea stars, brittle stars and sea squirts. Predators are clapper rails, diamondback turtles, water moccasins, raccoons and killifishes. Bacteria and fungi initially break down the leaf litter (decomposition). | |
| + | In the tree '''canopy''', vertebrate fauna and birds are common. Examples of birds are pelicans, wood ibises, herons, egrets and roseate spoonbills (Hogarth, 1999 <ref name=H99/>). | ||
| − | + | {| style= border="0" align="center" | |
| − | + | |- | |
| + | | valign="top" align=centre| | ||
| + | [[File: Uca lacteal 1.jpg|right|thumb|200px|caption|Fiddler crab ''Uca lacteal'' <ref>http://www.fiddlercrab.info/u_lactea.html</ref>.]] | ||
| + | | valign="top" align=centre| | ||
| + | [[File:Egretta alba.jpg|thumb|200px|Egret ''Egretta alba'' <ref>http://en.wikipedia.org/wiki/Snowy_Egret</ref>.]] | ||
| + | | valign="top"| | ||
| + | [[File:Agkistrodon contortrix.png|thumb|200px|Water moccasin ''Agkistrodon contortrix'' <ref>http://en.wikipedia.org/wiki/Agkistrodon_contortrix</ref>.]] | ||
| + | | valign="top"| | ||
| + | [[File:Diamondback turtle.jpg|thumb|200px|Diamondback turtle ''Malaclemys terrapin'' <ref>http://en.wikipedia.org/wiki/Diamondback_turtle</ref>.]] | ||
| + | |} | ||
| − | + | ==Coastal protection== | |
| − | + | Coastal protection is an important ecosystem service provided by mangroves. It has been estimated that mangroves protect millions of people from flooding every year, notably in densely populated areas of southeast Asia and reduce the costs of flood damages worldwide (Worthington and Spalding, 2018<ref name=WS>Worthington, T. and Spalding, M. 2018. Mangrove Restoration Potential: A global map highlighting a critical opportunity. Report, 26 October 2018. doi:10.17863/CAM.39153</ref>). The global flood protection benefits of mangroves has been estimated at more than $US 65 billion per year<ref>Menendez, P., Losada, I.J., Torres-Ortega, S., Narayan, S. and Beck, M.W. 2020. The global | |
| + | flood protection benefits of mangroves. Sci. Rep. 10, 4404</ref>. | ||
| + | Mangrove forests efficiently dissipate the energy of incident waves. Wave damping of 0.2 – 1.2 m has been observed per 100 m forest width, depending in particular on vegetation density (approximately linearly<ref>Kelty, K., Tomiczek, T., Cox, D.T., Lomonaco, P. and Mitchell, W. 2022. Prototype-Scale Physical Model of Wave Attenuation Through a Mangrove Forest of Moderate Cross-Shore Thickness: LiDAR-Based Characterization and Reynolds Scaling for Engineering With Nature. Front. Mar. Sci. 8: 780946</ref>) and vegetation type<ref name=H14/>. Mangroves also help to mitigate the threat of inundation and devastation by storm surges and cyclones, building a living seawall that can slow or halt erosion, and temper flood flows driven by storm surges. Storm surge attenuation depends on the width and density of the mangrove belt, with a stronger effect of short trees compared to tall trees; an order of magnitude estimate of the observed decrease of storm surge height in the South Florida mangrove zone for Hurricane Wilma (2005) was 0.2 m per km forest width (Chen et al., 2021<ref>Chen, Q., Li, Y., Kelly, D.M., Zhang, K., Zachry, B. and Rhome, J. 2021. Improved modeling of the role of mangroves in storm surge attenuation. Estuarine, Coastal and Shelf Science 260, 107515</ref>). | ||
| − | The | + | The coastal protection function is further enhanced by sediment trapping in mangrove forests. Observed annual accretion rates from external sources are of the order of one up to a few cm<ref>Chaudhuri, P., Chaudhuri, S. and Ghosh, R. 2019. The Role of Mangroves in Coastal and Estuarine Sedimentary Accretion in Southeast Asia. Sedimentary Processes - Examples from Asia, Turkey and Nigeria. IntechOpen DOI: http://dx.doi.org/10.5772/intechopen.85591</ref>. Moreover, thick litter layers build up over time due to accumulation of decaying organic matter, such as leaf and root litter and the formation of agal mats. However, in spite of substantial mineral and organic accretion, the observed net surface elevation change is much smaller - of the order of one or a few mm annually, generally positive and sometimes negative - due to subsidence and compaction processes<ref>Krauss, K.W., Cahoon, D.R., Allen, J.A., Ewel, K.C., Lynch, J.C. and Cormier, N. 2010. Surface elevation change and susceptibility of different mangrove zones to sea-level rise on Pacific high islands of Micronesia Ecosystems 13: 129–143</ref>. |
| + | ==Other ecosystem services== | ||
| + | * Carbon sequestration. Deposition of plant litter and woody debris, root accumulation and algal mat development contribute to carbon storage. Belowground biomass represents 85% of the total biomass in many mangrove forests (Kauffman et al., 2020<ref>Kauffman, J.B., Adame, M.F., Arifanti, V.B., Schile-Beers, L.M., Bernardino, A.F.,Bhomia, R.K., Donato, D.C., Feller, I.C., Ferreira, T.O., Jesus Garcia, M.d.C., MacKenzie, R.A., Megonigal, J.P., Murdiyarso, D., Simpson, L. and Hernandez Trejo, H. 2020. Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 90, e01405</ref>). Alongi (2020<ref>Alongi, D.M. 2020. Global significance of mangrove blue carbon in climate change mitigation. Science 2020, 2, 57</ref>) estimated the carbon burial by mangroves at about 180 gC/m/year, yielding a global carbon sequestration of about 15 million tonnes C /year. This is of the order of 1% of the global carbon sequestration by forests. However, according to Wang et al. (2021<ref name=W21>Wang, F., Sanders, C.J., Santos, I.R., Tang, J., Schurech, M., Kirwan, M.L., Kopp, R.E., Zhu, K., Li, X., Yuan, J., Liu, W. and Li, Z. 2021. Global blue carbon accumulation in tidal wetlands increases with climate change. National Science Review 8: nwaa296</ref>), this figure is underestimated by a factor 2-3 because the burial rate in the tropics is much faster than average (i.e. of the order of 400 gC/m/year). Carbon sequestration by mangroves may even be substantially larger than this burial estimate when taking into account the outwelling of dissolved inorganic carbon (DIC) to the deep sea, see [[Blue carbon sequestration]]. | ||
| + | * Food production. Mangrove communities are recognized as highly productive ecosystems that provide large quantities of organic matter to adjacent coastal waters in the form of detritus and live animals (fish, shellfish). The detritus serves as a nutrient source and is the base of an extensive food web in which organisms of commercial importance take part. In addition, mangrove ecosystems serve as shelter, feeding, and breeding zones for crustaceans, mollusks, fish of commercial importance, and resident and migratory birds (Holguin et al., 2001<ref name=H01>Holguin, G., Vazquez, P. and Bashan, Y. 2001. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fertil. Soils 33: 265–278</ref>). | ||
| + | * Denitrification. Anaerobic denitrification and nitrogen-fixation by certain bacteria and cyanobacteria associated with mangrove mud and with above-ground root systems can improve water quality from wastewater inputs (Romanacha et al., 2018<ref name=R18>Romanacha, S.S., DeAngelis, D.L., Koh, H.L., Li, Y., Teh, S.Y., Barizan, R.S.R. and Zhai, L. 2018. Conservation and restoration of mangroves: Global status, perspectives, and prognosis. Ocean and Coastal Management 154: 72–82</ref>). | ||
| − | |||
| + | ==Threats== | ||
| − | Mangroves | + | Mangroves are threatened in their existence by several causes, generally related to human activities. |
| + | * Variations in river and surface run-off, that deprive tropical coastal deltas of fresh water and sediment, entails reductions of species diversity and organic production. This results in alterations of both the terrestrial and aquatic [[food web]] and reduces habitats available to species of higher trophic levels. | ||
| + | * Soil reclamation for agriculture and aquaculture reduces regional biodiversity due to loss of mangrove [[habitat]]s. Many mangrove forests worldwide have been cleared to make way for shrimp aquaculture, with a strong negative impact on coastal safety and biodiversity. | ||
| + | * Clearcutting mangrove forest and replacement with dikes creates ponds with [[anoxic]] water that increases the level of sulphide in the soil and increases the pH leading to major shrimp losses. | ||
| + | * Another negative impact of humans on the mangrove [[habitat]] is the use of pesticides and fertilizers. The products that are used in the upstream agriculture end up in the water around the mangroves. This causes an increased nutrient concentration, especially [[nitrogen]] and [[phosphorus]]. These nutrients cause oxygen depletion in the water and promote the growth of [[algae]]. As a result, the ecosystems will be no longer in equilibrium. See also [[Possible consequences of eutrophication]]. | ||
| + | * A further issue is the clearcutting of mangals for their hard wood. This wood is resistant against termites and therefore an important export product for building constructions in areas with large concentrations of termites. The wood can also be used for charcoal and fuelwood. The substrate will be no longer stable when the trees are cut away and erosion will result (Besset et al., 2019<ref name=B19>Besset, M., Gratiot, N., Anthony, E.J., Bouchette, F., Goichot, M. and Marchesiello, P. 2019. Mangroves and shoreline erosion in the Mekong River delta. Estuarine. Coastal Shelf Sci. 226, 106263</ref>). | ||
| + | * A similar effect as with the added fertilizers and pesticides is the use of mangroves for waste-water treatment. Nutrients are added to the water and the equilibrium in the [[food web]] is disturbed. Mangroves no longer can survive in this environment and die off. The organic matter, normally stored in the mangroves, will be transported to open water and increases the aquatic [[primary production]]. This results in a huge amount of [[phytoplankton]] and water turbidity. [[Coral reefs|Corals]] and [[seagrass]]es are strongly affected by these processes and will be deteriorated. | ||
| + | * Other coastal development activities influence on the quality of the water. Industry, [[tourism]] and port development involve land reclamation and [[dredging]]. This causes resuspension of the [[sediment]] and increases the water turbidity. Light penetration in the water will be limited causing damage to the mangroves. | ||
| + | * [[Oil spills|Spills]] of oil, toxic chemicals and dumping of waste into the water causes localized impacts on the mangroves. The introduction of [[Non-native species invasions|alien species]] by [[ballast water]] or from the hulls of vessels will also have negative effects on the mangrove [[habitats]]. These species will compete with indigenous species for space and food. | ||
| + | * Climate change. Because mangroves raise the soil level, it is believed that mangrove forests can keep pace with a moderate sea level rise. Elevation gain by physical and biological processes generally exceeds subsoil compaction by dewatering and organic matter decomposition. In some cases, net elevation rates of about 5 mm/year have been recorded (Krauss et al., 2014<ref name=K14>Krauss, K.W., McKee, K.L., Lovelock, C.E., Cahoon, D.R., Saintilan, N., Reef, R. and Chen, L. 2014. How mangrove forests adjust to rising sea level. New Phytologist 202: 19–34</ref>). Mangroves do not survive when the rate of sea level exceeds the soil raising capacity. Another threat is the impact of storms that may become more severe and more frequent so that periods for recovery will become shorter. Inland migration of mangroves in response to sea-level rise occurs where the landward margin of mangroves is unimpeded by artificial or natural barriers (Friess et al., 2019<ref name=F19>Friess, D.A., Rogers, K., Lovelock, C.E., Krauss, K.W., Hamilton, S.E., Lee, S.Y., Lucas, R., Primavera, J., Rajkaran, A. and Shi, S. 2019. The state of the world’s mangrove forests: past, present and future. Annu. Rev. Environ. Resour. 44: 89–115</ref>; Bozi et al., 2021<ref>Bozi, B.S., Figueiredo, B.L., Rodrigues, E., Cohen, M.C.L., Pessenda, L.C.R., Alves, E.E.N., de Souza, A.V., Bendassolli, J.A., Macario, K., Azevedo, P. and Culligan, N. 2021. Impacts of sea-level changes on mangroves from southeastern Brazil during the Holocene and Anthropocene using a multi-proxy approach. Geomorphology 390, 107860</ref>). | ||
| − | == | + | ==Mangrove loss== |
| − | + | Worldwide, a total area of almost 10,000 km<sup>2</sup> (about 7%) of mangrove has been lost between 1996 and 2016, while an estimated 1,400 km2 of remaining mangrove forests are identified as degraded (Worthington and Spalding, 2018<ref name=WS>Worthington, T. and Spalding, M. 2018. Mangrove Restoration Potential: A global map highlighting a critical opportunity. Report, 26 October 2018. doi:10.17863/CAM.39153</ref>). Mangrove losses continue on every continent, although rates of loss have declined considerably from 1 – 3% in the late 20th century to 0.3 – 0.6% in the early 21st century (Friess et al., 2019<ref name=F19>Friess, D.A., Rogers, K., Lovelock, C.E., Krauss, K.W., Hamilton, S.E., Lee, S.Y., Lucas, R., Primavera, J., Rajkaran, A. and Shi, S. 2019. The state of the world’s mangrove forests: past, present and future. Annu. Rev. Environ. Resour. 44: 89–115</ref>). The main causes of mangrove loss are transformation of forests into economic land use such as aquaculture and agriculture, wood production, and (urban) infrastructure. | |
| + | There is little chance of spontaneous mangrove regeneration in the deforested lands (Coppejans<ref name=E/>; UNEP, 2006<ref>UNEP. 2006. In the frontline: shoreline protection and other ecosystem services from mangroves and coral reefs. p.36</ref>), due to increased wave agitation and soil erosion (Winterwerp et al. 2013<ref>Winterwerp, J., Erftemeijer, P., Suryadiputra, N., Van Eijk, P. and Zhang, L. 2013. Defining eco-morphodynamic requirements for rehabilitating eroding mangrove-mud coasts. Wetlands 33: 515–526</ref>). Non-native plant invasions may also restrict the re-growth of mangroves (Romanacha et al. 2018<ref name=R18/>). | ||
| − | |||
| + | ==Community participation in mangrove management== | ||
| − | [[ | + | There is ample evidence that mangrove forests are better managed with the involvement and active participation of local communities<ref name=R16>Rotich, B., Mwangi, E. and Lawry, S. 2016. Where land meets the sea: a global review of the governance and tenure dimensions of coastal mangrove forests. Bogor, Indonesia: CIFOR; Washington, DC: USAID Tenure and Global Climate Change Program</ref>. Mangroves are a natural resource that can provide coastal communities with essential livelihoods. Government policies aimed at conserving mangrove forests by denying access to this resource are often doomed to failure. Local communities, given the right regulatory context, are often best placed to manage these local ecosystems, as their close proximity permits them to develop rules that match the resource and social situation<ref name=R16/>. Uncontrolled exploitation of mangroves can lead to the loss of the natural resource due to the so-called '[[The tragedy of the commons: Is the Newfoundland's cod crisis a good example?|tragedy of the commons]]'. Controlled exploitation, cutting down less healthy trees ('thinning') or the oldest trees ('selective harvesting') can even benefit the mangrove forest if young seedlings are planted in the cleared areas. Studies have reported enhanced stand biomass, improved growth conditions, increased carbon sequestration and improved forest productivity<ref>Phong, N.T. and Nuong, C.T. 2023. Thinning, selective harvesting and mangrove protection forests: Lessons learned and recommendations from the Vietnamese Mekong Delta. Estuarine, Coastal and Shelf Science 288, 108345</ref>. Controlled exploitation, based on enforced rules, can commit local communities to participate in sustainable mangrove management. Not all mangrove coasts can be managed in this way; stable or accreting coastlines are a requirement<ref>Luom, T.T., Phong, N.T., Smithers, S. and Tai, V.T. 2021. Protected mangrove forests and aquaculture development for livelihoods. Ocean and Coastal Management 205, 105553</ref>. |
| − | + | ==Mangrove [[Ecosystem restoration|restoration]] and [[Ecosystem rehabilitation|rehabilitation]]== | |
| − | |||
| − | |||
| + | ===Causes of failure=== | ||
| + | Mangrove replanting projects have been undertaken in many places worldwide (Friess et al., 2019<ref name=F19/>). | ||
| + | However, few replanting programs have proven successful. A first major issue is that many [[Ecosystem rehabilitation|rehabilitation]] projects start planting before studying the original cause of mangrove loss to find out why there is no natural regeneration on site (Lewis, 2005<ref name=L5>Lewis III, R.R. 2005. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24: 403–418</ref>). Often essential conditions are not met because previous reclamations and interventions may have rendered the site less suitable for mangrove regeneration. For example, compacted mudflats often have permanently saturated soil with poor drainage, leading to anoxic and potentially acidic soil (Holguin et al., 2001<ref name=H01>Holguin, G., Vazquez, P. and Bashan, Y. 2001. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fertil. Soils 33: 265–278</ref>). A second major issue is that species chosen for replanting may not be appropriate for the currently prevailing site conditions. Site conditions include: salinity, soil type, soil anoxia, sulphate levels, nutrient levels, pH, wave energy, temperature, light levels, inundation regimes, tides and wind distribution of propagules and seeds (Wodehouse, 2019<ref name=W19>Wodehouse, D.C. and Rayment, M.B. 2019. Mangrove area and propagule number planting targets produce sub-optimal rehabilitation and afforestation outcomes. Estuar. Coast. Shelf Sci. 222: 91–102</ref>). | ||
| + | Other reported reasons for failure include: poor planting method, lack of aftercare (e.g. weeding) and monitoring, fresh water availability, lack of drainage and sediment availability and high wave energy (i.e. inappropriate site choice). Unfavorable biological conditions range from limited seed availability, insufficient seed transport capacity, to adverse biotic activity such as barnacle infestation, predation by crabs and bioturbation by worms, burying small seedlings. A further impediment is the large quantity of household waste, most notably plastic. Plastic getting stuck to seedlings increase the chance of uprooting, and covering pneumatophores causes deformation of the roots, while the tree attempts to outgrow the suffocating material (Winterwerp et al., 2020<ref name=W20>Winterwerp, J.C., Albers, T., Anthony, E.J., Friess, D.A., Mancheno, A.G., Moseley, K., Muhari, A., Naipal, S., Noordermeer, J., Oost, A., Saengsupavanich, C., Tas, S.A.J., Tonneijck, F.H., Wilms, T., Van Bijsterveldt, C., Van Eijk, P., Van Lavieren, E. and Van Wesenbeeck, B.K. 2020. Managing erosion of mangrove-mud coasts with permeable dams – lessons learned. Ecological Engineering 158, 106078</ref>). | ||
| − | + | ===Principles of Ecological Mangrove Restoration (EMR)=== | |
| − | + | EMR aims at restoring the favorable habitat conditions for mangroves, and generally no planting is done (Lewis, 2005<ref name=L5/>). In this way, EMR strives for natural zonation and optimized species site matching. This results in fast growth of the forest and high survival rates. If abiotic conditions are favorable, mangroves generally recruit spontaneously and grow naturally. This is often preferable to planting, as natural recolonization can be very fast if conditions are favorable, whereas up to 85% of planting efforts fail. After restoration, it may take only 5 years to obtain a forest's maximum coastal protection capacity. Additionally, such capacity appears to be maintained throughout the forest's lifetime with minor variations, provided the forest is not affected by any conditions altering its health, such as extreme wave events or anthropogenic action that may modify its habitat (Maza et al., 2021<ref>Maza, M., Lara, J.L. and Losada, I.J. 2021. Predicting the evolution of coastal protection service with mangrove forest age. Coastal Engineering 168, 103922</ref>). | |
| − | |||
| − | |||
| − | |||
| + | If regeneration through natural [[recruitment]] is not an option, species must be carefully selected for planting. Planting mixed species produces a richer mangrove community and higher plant success rates, provided it is accompanied by biotic and abiotic research, while often also requiring hydrological restoration (Primavera et al., 2012<ref name=P12>Primavera, J.H., Savaris, J.P., Bajoyo, B.E., Coching, J.D., Curnick, D.J., Golbeque, R.L., Guzman, A.T., Henderin, J.Q., Joven, R.V., Loma, R.A. and Koldewey, H.J. 2012. Manual on Community-based Mangrove Rehabilitation, Mangrove Manual Series No.1 London. 240 pp.</ref>). | ||
| − | == | + | Sites that have been altered by previous reclamation and interventions require prior physical [[Ecosystem restoration|restoration]]. Altered physical conditions include: wave climate, currents, flushing, sedimentation, and drainage. Wave conditions can be restored by the installation of permeable dams, consisting of horizontally placed brushwood, which damp the waves, and vertical poles to hold the brushwood. Bamboo is a suitable material for the construction of such dams. Detailed instructions for the construction of impermeable dams are given by Winterwerp et al. (2020<ref name=W20/>). The configuration of the dams must be designed such that the original sedimentation regime is also restored, as mangrove degradation may cause accreting/stable coastlines turning towards an erosive state (Besset et al., 2019<ref name=B19/>). Disturbance of the fine sediment balance is a also an important cause of the poor success of [[Ecosystem rehabilitation|rehabilitation]] efforts on eroding coastlines (Winterwerp et al., 2020<ref name=W20/>). Newly formed mud flats should be protected from fishing and other bottom-disturbing activities, as frequent stirring up the fresh deposits will prevent mangrove [[recruitment]]. Project outcomes can further be improved by restoring appropriate hydrological connectivity with good tidal flushing and drainage (Lewis, 2005<ref name=L5/>). |
| − | + | An alternative method may exist to create conditions that stimulate mangrove regeneration in areas where coarse sediment (e.g. shell sand) is available. If the seabed in the vicinity contains such sediments, it can be winnowed for the stabilization or supply of possibly existing chenier remnants or otherwise for the construction of artificial cheniers some distance seaward of the shoreline. The protection provided by cheniers appears generally very beneficial for the development of mangrove forests, especially on microtidal coasts<ref>van Bijsterveldt, C.E.J., van der Wal, D., Mancheno, A.G., Fivash, G.S., Helmi, M. and Bouma, T.J. 2023. Can cheniers protect mangroves along eroding coastlines? – The effect of contrasting foreshore types on mangrove stability. Ecological Engineering 187, 106863</ref>, see the article [[Chenier]]. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | [[Ecosystem rehabilitation|Rehabilitation]] of mangroves and their habitat is rarely successful without the involvement of local stakeholders. Socio-economic aspects should be included in restoration projects so that local communities benefit from sustainable mangrove use. It is essential to introduce sustainable economic activities alongside mangrove [[Ecosystem restoration|restoration]], such as sustainable aquaculture and integrated mangrove-aquaculture schemes, fisheries, eco-tourism and non-timber forest products (Primavera et al., 2012<ref name=P12/>). | ||
| − | + | ===Cost-benefit of mangrove restoration=== | |
| − | + | Estimates of costs and benefits of mangrove restoration widely diverge<ref name=S21>Su, J., Friess, D.A. and Gasparatos, A. 2021. A meta-analysis of the ecological and economic outcomes of mangrove restoration. Nature Communications 12: 5050</ref>. High-rated benefits are fisheries, timber, waste water treatment and coastal protection. An average estimate is 20,000 US$ per ha and per year, within a range of 500-100,000. This large range can (partly) be explained by differences in valuation methods and site-specific factors such as vegetation characteristics (e.g. species, age, tree density, height, root), sediments (e.g. depth, nutrients) and various environmental variables (e.g. location, salinity, debris). | |
| + | Restoration costs reported in the literature fall in an even wider range of US$ 500-400,000 per ha, which can be (partly) explained by the type of engineering interventions, planting vs. natural regeneration and labour costs. Comparing the figures for restoration (per ha) and for benefits (per ha and per year), in almost every case efficient restoration measures yield benefits that greatly exceed the costs<ref name=S21/>. | ||
==Further reading== | ==Further reading== | ||
| + | Spalding, M. 2011. World Atlas of Mangroves: Mark Spalding, Mami Kainuma and Lorna Collins (eds.) London, Washington D.C.: Earthscan 2010. ISBN 978-1-84407-657-4 | ||
| + | |||
| + | |||
| + | ==Related articles== | ||
| + | :[[Marine habitats and ecosystems]] | ||
| + | :[[ Blue carbon revenues of nature-based coastal protection]] | ||
| + | :[[Characteristics of sedimentary shores]] | ||
| + | :[[Potential Impacts of Sea Level Rise on Mangroves]] | ||
| + | :[[Chenier]] | ||
| + | :[[Coastal mud belt]] | ||
| − | |||
| − | http://www.vliz.be/vmdcdata/mangroves/index.php | + | ==External links== |
| + | * [http://www.fao.org/forestry/site/mangrove/en/ UN Mangrove Management] | ||
| + | * http://www.ozcoasts.gov.au/indicators/mangrove_areas.jsp | ||
| + | * http://www.vliz.be/vmdcdata/mangroves/index.php | ||
| + | * http://www.glomis.com | ||
| + | * https://en.wikipedia.org/wiki/Mangrove | ||
| − | |||
==References== | ==References== | ||
| Line 137: | Line 218: | ||
{{author | {{author | ||
| − | |AuthorID= | + | |AuthorID=16323 |
| − | |AuthorFullName= | + | |AuthorFullName=Töpke, Katrien |
|AuthorName=Ktopke}} | |AuthorName=Ktopke}} | ||
| − | [[Category: | + | |
| − | [[Category: | + | {{Review |
| − | [[Category: | + | |name=Job Dronkers|AuthorID=120| |
| − | [[Category: | + | }} |
| + | |||
| + | |||
| + | [[Category:Coastal and marine ecosystems]] | ||
| + | [[Category:Coastal and marine human activities and uses]] | ||
| + | [[Category:Coastal and marine habitats]] | ||
| + | [[Category:Mangroves]] | ||
| + | [[Category:Soft coastal interventions]] | ||
Revision as of 15:37, 5 December 2023
This article describes the habitat of the mangrove forests. It is one of the subcategories within the section dealing with the biodiversity of marine habitats and ecosystems. It provides an overview of the characteristics, distribution, biota, functioning and adaptation to habitat conditions. An introduction is given to management aspects, discussing threats, conservation and rehabilitation of mangrove forests.
Contents
- 1 Introduction
- 2 Requirements for development
- 3 Distribution
- 4 Functioning and adaptations
- 5 Biota
- 6 Coastal protection
- 7 Other ecosystem services
- 8 Threats
- 9 Mangrove loss
- 10 Community participation in mangrove management
- 11 Mangrove restoration and rehabilitation
- 12 Further reading
- 13 Related articles
- 14 External links
- 15 References
Introduction
Mangroves are the only trees that are capable of thriving in salt water. They form unique intertidal forests at the edge of land and sea, see Fig. 1. They are represented on all continents with tropical and subtropical coasts, i.e. North and South America, Africa and Middle-East, Asia and Oceania (incl. Australia). [1]
Mangrove forests or mangals are a type of intertidal wetland ecosystems. The word mangrove is derived from the Portugese word mangue which means “tree” and the English word grove which is used for trees and shrubs that are found in shallow, sandy or muddy areas (Karleskint, 1998[2]). They replace salt marshes in tropical and subtropical regions. They are salt-tolerant forested wetlands at the interface between the terrestrial landscape and the marine environment. The dominant vegetation are several species of mangrove: woody trees and shrubs with a thick, partially exposed network of roots that grow down from the branches into the water and sediment. They settle where there is little wave action and where muddy sediments accumulate. While growing, mangal forests further reduce waves and increase sedimentation. Wave energy reduction can be greater than 50% on average and increases with increasing offshore wave heights (Horstman et al., 2014[3]). Mangals therefore fulfill an important coastal protection function. Mangroves are frequently associated with saline lagoons and are regularly found on protected sides of islands, atolls and tropical estuaries (Karleskint, 1998[2]).
Requirements for development
Requirements for the development of mangroves are:
- Average temperature of the coldest month higher than 20°C; the seasonal temperature range should not exceed 5°C. They are not resistant to freezing.
- A fine-grained substrate. Exceptions are the development of mangroves on corals, as for example in Papua New Guinea and Kenya.
- Shores must be free of strong wave action and strong tidal currents.
- Saline water; they are facultative halophytes.
- Deposition of sediments by small-moderate waves and tides.
Due to these requirements, a well-marked zonation is seen. Each of the zones is dominated by a different mangrove species and associated fauna and flora. Red mangroves (Rhizophora) are usually found closest to the edge of the water, where the greatest degree of tidal flooding occurs. More landwards are the black mangroves (Avicennia). These areas receive only shallow flooding during high tide. The upper limit of the mangroves is occupied with white mangroves and buttonwoods. The buttonwoods are not really a mangrove species, but are a transition species between the mangrove and the terrestrial vegetation.
Distribution
The area covered by mangroves worldwide is estimated at almost 150,000 km2 [4]. The distribution, density and species composition are determined by the water and air temperatures during the winter, exposure to wave action and tidal currents, the range of the tide, the type of sediment and the chemistry of the seawater. The global distribution of mangroves is shown in Fig. 2. The most highly developed and most species-rich mangals are found in Indonesia, Australia and Malaysia. Over the world, 54-70 species and hybrids in 20-27 genera and 16-19 families are found (Berness et al., 2001[5]). A species overview is given in the Mangrove Species Database [1].
Mangroves are almost exclusively tropical, but also occur in the subtropics. They do not tolerate frost, but can cope with air temperatures down to 5 °C. Their occurrence is most closely related to seawater temperature. The isotherm of 20 °C in winter is a good indicator of the distribution limit. Avicennia appears to be more tolerant of low temperatures than Rhizophora or Laguncularia. The number of species tends to decrease with distance from the equator. In the Southern Hemisphere, mangals generally occur further south on the eastern edges of landmasses than on the western side. This is due to the pattern of hot and cold ocean currents (Pinet, 1998[6]; Hogarth, 1999 [7]).
As a result of global warming, the area with a suitable settling climate for mangroves is currently expanding in the polar direction. Colonization of salt marshes has been observed along several continents in recent decades, especially by the most cold-tolerant mangrove genus Avicennia (Saintilan et al., 2014[8]).
Functioning and adaptations
Although mangrove species are unrelated taxonomically, they exhibit similar morphological, physiological and reproductive traits. Mangroves have several functions and adaptations for thriving in saline intertidal zones. They grow in an environment whose salinity ranges between freshwater and seawater. For this reason, they have to take up water against the osmotic pressure. To overcome the negative osmotic pressure, they generate a negative hydrostatic pressure (by transpiration processes). Roots or leaves exude salt, which make them tolerant to saline conditions. Even after most of the salts have been removed, concentration of chloride and sodium ions in the tissue is higher than in other plants. Salt is stored in vacuoles to protect enzymes that might otherwise be inhibited. The high cation concentrations are balanced by high non-ionic solutes in the cytoplasm. Several mangrove species deposit sodium and chloride in the bark of stems and roots. Other species deposit salt in senescent leaves, which later fall off the tree. Salt glands on the leaves also exude salt that forms crystals. In some species, fine hairs covering the lower leaf surface raise the secreted droplets of salt water away from the leaf surface in order to prevent osmotic withdrawal of water from the leaf tissues (Parida and Jha, 2010[9]).
Mangroves also need adaptations to conserve water. Leaves have a thick waxy cuticle (skin on the leaf) or dense hairs to reduce transpiration, or orientate their leaves to avoid the burning sun. Most evaporation loss occurs through stomata - pores in the leaves - so these are often sunken below the leaf surface where they are protected from drying winds. Leaves are also commonly succulent, storing water in fleshy internal tissue.
Because salt tolerance is costly, a greater relative root mass is needed to recover the demand for water. When it rains, drop roots, roots that descend from the branches, absorb the freshwater that runs down from the stem through a special superficial layer. In this way, no high-energy inverse osmosis is needed.
 Salt crystals on a mangrove leaf [10] |
 Extended root mass. Photo credit Eric Coppejans [11]. |
 Drop roots from branches [12] |
Deposits of fine grained (muddy and sandy) sediments lack oxygen (Hogarth, 1999[7]).
Mangroves therefore have to cope with anoxic conditions. The tissue of the plants requires oxygen for respiration which cannot diffuse sufficiently into soils that are waterlogged. Even if the surface water is saturated with oxygen, its concentration in the groundwater is too low. This is why mangroves develop various forms of aerial roots.
- Most of the roots branch off from the stem underground. One type of roots is the prop root (also called stilt root). This root diverges from the tree and anchors into the bottom to stabilize the tree in the soft, muddy substrate. The rhizophore has lenticels on the upper surface, large pores with a corky layer enabling the uptake of oxygen. Seawater cannot get into the lenticels. The tissue of the prop roots consists of aerenchyma and is connected with the lenticels. Through this aerenchyma, oxygen can be provided to the submerged parts of the tree. The roots that break at alternating places through the soil surface and submerge again form a knee root.
 Knee roots. Photo credit Eric Coppejans [11] |
 Prop roots growing from the lower trunk [10] |
- Another type of roots is a shallow, horizontal root that radiates outwards. The vertical root is called a pneumatophore and can be as high as several decimeters. These roots also have lenticels and aerenchyma. They can create a huge network of vertical roots. The horizontal root is called the cable root.
- The buttress root (also called plank root) is a root that covers the whole space between the upper part of the root and the bottom.
 Pneumatophores on cable roots [10]. |
 Buttress root (plank root). Photo credit Eric Coppejans [11]. |
 Propagules. Photo credit Eric Coppejans [11]. |
Observations of the mangrove species distribution along the Mekong delta coast showed that pneumatophore species (e.g., Avicennia alba) were preferentially found in accreting sites where soil was often inundated and oxygen-poor mud had accumulated. Stilt root species (e.g., Rhizophora apiculata) occurred mostly in erosion sites where stabilizing support is important. Knee root species (e.g., Bruguiera parviflora) dominated in higher sites with more stable substrate[13]. Avicennia marina, Rhizophora apiculata, Bruguiera gymnorrhiza and Xylocarpus granatum are species with high carbon sequestration capacity. A. marina and R. apiculata strive on sand-silt soils in the low-medium intertidal zone, while B. gymnorrhiza and X. granatum are often found on silt-clay soils in the high-intertidal zone, especially in estuaries[14].
Mangroves aid soil formation by trapping debris. Plank roots and pneumatophores accumulate sediments in protected sites and form mangrove peats; sediment trapping is a function of the volume of aboveground roots (Du et al., 2021[15]). Root production also contributes to soil elevation, even more than leaf, twig and branch litter, due to slow decomposition (Krauss et al., 2014[16]). Filamentous algae help to stabilize the fine sediments trapped by mangroves. They usually form a green-to-red mass over the substrate. They are also a filtering system for the land run-off and remove the terrestrial organic matter. They are very important habitats for many species of small fish, invertebrates and various epiflora and epifauna as well as larger birds. This is called a nursery function. The mangrove is a major producer of detritus that will contribute to offshore productivity in some seasons (Coppejans [17]).
Pollination of the trees is done by the wind or by organisms. All mangroves disperse their offspring by water. They produce unusually large propagating structures or propagules. The embryo initiates germination on the seed, still attached on the tree and further develops into a propagule. This phenomenon is known as vivipary.
Biota
Epiphytic micro-algae grow on the aerial roots of the trees and on the sediments. The algae are green ( Chlorophyta), brown (Phaeophyceae), red (Rhodophyta) and blue-green (Cyanophyta). The dense biomass on the aerial roots causes the water to remain on the pneumatophores; the lenticels are no longer functional and oxygen cannot penetrate into the roots. For this reason, the bark regularly falls off the root. This process is called decertification. Vertical zonation along a single pneumatophore occurs, but there is also a zonation from the upper limit of the mangroves to the lower limit.
Several invertebrate species are found in the mangrove ecosystem. Macrobenthic species are commonly or occasionally present because they are migrating or because they are living in adjacent environments. Crabs are keystone species in the mangrove ecosystem. This means that the presence of this animal in the community makes it possible for many other species to live there. The crabs go through their larval stages in the water beneath the mangroves. When they are mature, they crawl up on the mangroves and feed on the leaves. They can reach high densities and are crucial in the processing of leaf litter. Their burrowing activity modifies the micro-topography of the bottom and aerates the soil. This decreases the sulphide levels in the soil and positively influences the productivity of the trees. An example of a mangrove crab is the fiddler crab Uca lacteal.
An important bivalve is the purple oyster Lopha frons. This species encrusts the pneumatophores and prop roots. When the tide is high, barnacles and mussels compete with the oyster for space on the roots. Periwinkles also occur on the roots and stems, as well as on the shells of sedentary organisms attached on them. Snails are important in the turnover of the organic material. Other species that occur in the mangroves are tunicates, sponges, ants, hermit crabs, shrimps, fishes,… They are a source of nutrition for higher level predators. Species that cannot tolerate changing saline conditions can survive in the forest. These species are sea stars, brittle stars and sea squirts. Predators are clapper rails, diamondback turtles, water moccasins, raccoons and killifishes. Bacteria and fungi initially break down the leaf litter (decomposition). In the tree canopy, vertebrate fauna and birds are common. Examples of birds are pelicans, wood ibises, herons, egrets and roseate spoonbills (Hogarth, 1999 [7]).
 Fiddler crab Uca lacteal [18]. |
 Egret Egretta alba [19]. |
 Water moccasin Agkistrodon contortrix [20]. |
 Diamondback turtle Malaclemys terrapin [21]. |
Coastal protection
Coastal protection is an important ecosystem service provided by mangroves. It has been estimated that mangroves protect millions of people from flooding every year, notably in densely populated areas of southeast Asia and reduce the costs of flood damages worldwide (Worthington and Spalding, 2018[22]). The global flood protection benefits of mangroves has been estimated at more than $US 65 billion per year[23].
Mangrove forests efficiently dissipate the energy of incident waves. Wave damping of 0.2 – 1.2 m has been observed per 100 m forest width, depending in particular on vegetation density (approximately linearly[24]) and vegetation type[3]. Mangroves also help to mitigate the threat of inundation and devastation by storm surges and cyclones, building a living seawall that can slow or halt erosion, and temper flood flows driven by storm surges. Storm surge attenuation depends on the width and density of the mangrove belt, with a stronger effect of short trees compared to tall trees; an order of magnitude estimate of the observed decrease of storm surge height in the South Florida mangrove zone for Hurricane Wilma (2005) was 0.2 m per km forest width (Chen et al., 2021[25]).
The coastal protection function is further enhanced by sediment trapping in mangrove forests. Observed annual accretion rates from external sources are of the order of one up to a few cm[26]. Moreover, thick litter layers build up over time due to accumulation of decaying organic matter, such as leaf and root litter and the formation of agal mats. However, in spite of substantial mineral and organic accretion, the observed net surface elevation change is much smaller - of the order of one or a few mm annually, generally positive and sometimes negative - due to subsidence and compaction processes[27].
Other ecosystem services
- Carbon sequestration. Deposition of plant litter and woody debris, root accumulation and algal mat development contribute to carbon storage. Belowground biomass represents 85% of the total biomass in many mangrove forests (Kauffman et al., 2020[28]). Alongi (2020[29]) estimated the carbon burial by mangroves at about 180 gC/m/year, yielding a global carbon sequestration of about 15 million tonnes C /year. This is of the order of 1% of the global carbon sequestration by forests. However, according to Wang et al. (2021[30]), this figure is underestimated by a factor 2-3 because the burial rate in the tropics is much faster than average (i.e. of the order of 400 gC/m/year). Carbon sequestration by mangroves may even be substantially larger than this burial estimate when taking into account the outwelling of dissolved inorganic carbon (DIC) to the deep sea, see Blue carbon sequestration.
- Food production. Mangrove communities are recognized as highly productive ecosystems that provide large quantities of organic matter to adjacent coastal waters in the form of detritus and live animals (fish, shellfish). The detritus serves as a nutrient source and is the base of an extensive food web in which organisms of commercial importance take part. In addition, mangrove ecosystems serve as shelter, feeding, and breeding zones for crustaceans, mollusks, fish of commercial importance, and resident and migratory birds (Holguin et al., 2001[31]).
- Denitrification. Anaerobic denitrification and nitrogen-fixation by certain bacteria and cyanobacteria associated with mangrove mud and with above-ground root systems can improve water quality from wastewater inputs (Romanacha et al., 2018[32]).
Threats
Mangroves are threatened in their existence by several causes, generally related to human activities.
- Variations in river and surface run-off, that deprive tropical coastal deltas of fresh water and sediment, entails reductions of species diversity and organic production. This results in alterations of both the terrestrial and aquatic food web and reduces habitats available to species of higher trophic levels.
- Soil reclamation for agriculture and aquaculture reduces regional biodiversity due to loss of mangrove habitats. Many mangrove forests worldwide have been cleared to make way for shrimp aquaculture, with a strong negative impact on coastal safety and biodiversity.
- Clearcutting mangrove forest and replacement with dikes creates ponds with anoxic water that increases the level of sulphide in the soil and increases the pH leading to major shrimp losses.
- Another negative impact of humans on the mangrove habitat is the use of pesticides and fertilizers. The products that are used in the upstream agriculture end up in the water around the mangroves. This causes an increased nutrient concentration, especially nitrogen and phosphorus. These nutrients cause oxygen depletion in the water and promote the growth of algae. As a result, the ecosystems will be no longer in equilibrium. See also Possible consequences of eutrophication.
- A further issue is the clearcutting of mangals for their hard wood. This wood is resistant against termites and therefore an important export product for building constructions in areas with large concentrations of termites. The wood can also be used for charcoal and fuelwood. The substrate will be no longer stable when the trees are cut away and erosion will result (Besset et al., 2019[33]).
- A similar effect as with the added fertilizers and pesticides is the use of mangroves for waste-water treatment. Nutrients are added to the water and the equilibrium in the food web is disturbed. Mangroves no longer can survive in this environment and die off. The organic matter, normally stored in the mangroves, will be transported to open water and increases the aquatic primary production. This results in a huge amount of phytoplankton and water turbidity. Corals and seagrasses are strongly affected by these processes and will be deteriorated.
- Other coastal development activities influence on the quality of the water. Industry, tourism and port development involve land reclamation and dredging. This causes resuspension of the sediment and increases the water turbidity. Light penetration in the water will be limited causing damage to the mangroves.
- Spills of oil, toxic chemicals and dumping of waste into the water causes localized impacts on the mangroves. The introduction of alien species by ballast water or from the hulls of vessels will also have negative effects on the mangrove habitats. These species will compete with indigenous species for space and food.
- Climate change. Because mangroves raise the soil level, it is believed that mangrove forests can keep pace with a moderate sea level rise. Elevation gain by physical and biological processes generally exceeds subsoil compaction by dewatering and organic matter decomposition. In some cases, net elevation rates of about 5 mm/year have been recorded (Krauss et al., 2014[16]). Mangroves do not survive when the rate of sea level exceeds the soil raising capacity. Another threat is the impact of storms that may become more severe and more frequent so that periods for recovery will become shorter. Inland migration of mangroves in response to sea-level rise occurs where the landward margin of mangroves is unimpeded by artificial or natural barriers (Friess et al., 2019[34]; Bozi et al., 2021[35]).
Mangrove loss
Worldwide, a total area of almost 10,000 km2 (about 7%) of mangrove has been lost between 1996 and 2016, while an estimated 1,400 km2 of remaining mangrove forests are identified as degraded (Worthington and Spalding, 2018[22]). Mangrove losses continue on every continent, although rates of loss have declined considerably from 1 – 3% in the late 20th century to 0.3 – 0.6% in the early 21st century (Friess et al., 2019[34]). The main causes of mangrove loss are transformation of forests into economic land use such as aquaculture and agriculture, wood production, and (urban) infrastructure. There is little chance of spontaneous mangrove regeneration in the deforested lands (Coppejans[17]; UNEP, 2006[36]), due to increased wave agitation and soil erosion (Winterwerp et al. 2013[37]). Non-native plant invasions may also restrict the re-growth of mangroves (Romanacha et al. 2018[32]).
Community participation in mangrove management
There is ample evidence that mangrove forests are better managed with the involvement and active participation of local communities[38]. Mangroves are a natural resource that can provide coastal communities with essential livelihoods. Government policies aimed at conserving mangrove forests by denying access to this resource are often doomed to failure. Local communities, given the right regulatory context, are often best placed to manage these local ecosystems, as their close proximity permits them to develop rules that match the resource and social situation[38]. Uncontrolled exploitation of mangroves can lead to the loss of the natural resource due to the so-called 'tragedy of the commons'. Controlled exploitation, cutting down less healthy trees ('thinning') or the oldest trees ('selective harvesting') can even benefit the mangrove forest if young seedlings are planted in the cleared areas. Studies have reported enhanced stand biomass, improved growth conditions, increased carbon sequestration and improved forest productivity[39]. Controlled exploitation, based on enforced rules, can commit local communities to participate in sustainable mangrove management. Not all mangrove coasts can be managed in this way; stable or accreting coastlines are a requirement[40].
Mangrove restoration and rehabilitation
Causes of failure
Mangrove replanting projects have been undertaken in many places worldwide (Friess et al., 2019[34]). However, few replanting programs have proven successful. A first major issue is that many rehabilitation projects start planting before studying the original cause of mangrove loss to find out why there is no natural regeneration on site (Lewis, 2005[41]). Often essential conditions are not met because previous reclamations and interventions may have rendered the site less suitable for mangrove regeneration. For example, compacted mudflats often have permanently saturated soil with poor drainage, leading to anoxic and potentially acidic soil (Holguin et al., 2001[31]). A second major issue is that species chosen for replanting may not be appropriate for the currently prevailing site conditions. Site conditions include: salinity, soil type, soil anoxia, sulphate levels, nutrient levels, pH, wave energy, temperature, light levels, inundation regimes, tides and wind distribution of propagules and seeds (Wodehouse, 2019[42]). Other reported reasons for failure include: poor planting method, lack of aftercare (e.g. weeding) and monitoring, fresh water availability, lack of drainage and sediment availability and high wave energy (i.e. inappropriate site choice). Unfavorable biological conditions range from limited seed availability, insufficient seed transport capacity, to adverse biotic activity such as barnacle infestation, predation by crabs and bioturbation by worms, burying small seedlings. A further impediment is the large quantity of household waste, most notably plastic. Plastic getting stuck to seedlings increase the chance of uprooting, and covering pneumatophores causes deformation of the roots, while the tree attempts to outgrow the suffocating material (Winterwerp et al., 2020[43]).
Principles of Ecological Mangrove Restoration (EMR)
EMR aims at restoring the favorable habitat conditions for mangroves, and generally no planting is done (Lewis, 2005[41]). In this way, EMR strives for natural zonation and optimized species site matching. This results in fast growth of the forest and high survival rates. If abiotic conditions are favorable, mangroves generally recruit spontaneously and grow naturally. This is often preferable to planting, as natural recolonization can be very fast if conditions are favorable, whereas up to 85% of planting efforts fail. After restoration, it may take only 5 years to obtain a forest's maximum coastal protection capacity. Additionally, such capacity appears to be maintained throughout the forest's lifetime with minor variations, provided the forest is not affected by any conditions altering its health, such as extreme wave events or anthropogenic action that may modify its habitat (Maza et al., 2021[44]).
If regeneration through natural recruitment is not an option, species must be carefully selected for planting. Planting mixed species produces a richer mangrove community and higher plant success rates, provided it is accompanied by biotic and abiotic research, while often also requiring hydrological restoration (Primavera et al., 2012[45]).
Sites that have been altered by previous reclamation and interventions require prior physical restoration. Altered physical conditions include: wave climate, currents, flushing, sedimentation, and drainage. Wave conditions can be restored by the installation of permeable dams, consisting of horizontally placed brushwood, which damp the waves, and vertical poles to hold the brushwood. Bamboo is a suitable material for the construction of such dams. Detailed instructions for the construction of impermeable dams are given by Winterwerp et al. (2020[43]). The configuration of the dams must be designed such that the original sedimentation regime is also restored, as mangrove degradation may cause accreting/stable coastlines turning towards an erosive state (Besset et al., 2019[33]). Disturbance of the fine sediment balance is a also an important cause of the poor success of rehabilitation efforts on eroding coastlines (Winterwerp et al., 2020[43]). Newly formed mud flats should be protected from fishing and other bottom-disturbing activities, as frequent stirring up the fresh deposits will prevent mangrove recruitment. Project outcomes can further be improved by restoring appropriate hydrological connectivity with good tidal flushing and drainage (Lewis, 2005[41]).
An alternative method may exist to create conditions that stimulate mangrove regeneration in areas where coarse sediment (e.g. shell sand) is available. If the seabed in the vicinity contains such sediments, it can be winnowed for the stabilization or supply of possibly existing chenier remnants or otherwise for the construction of artificial cheniers some distance seaward of the shoreline. The protection provided by cheniers appears generally very beneficial for the development of mangrove forests, especially on microtidal coasts[46], see the article Chenier.
Rehabilitation of mangroves and their habitat is rarely successful without the involvement of local stakeholders. Socio-economic aspects should be included in restoration projects so that local communities benefit from sustainable mangrove use. It is essential to introduce sustainable economic activities alongside mangrove restoration, such as sustainable aquaculture and integrated mangrove-aquaculture schemes, fisheries, eco-tourism and non-timber forest products (Primavera et al., 2012[45]).
Cost-benefit of mangrove restoration
Estimates of costs and benefits of mangrove restoration widely diverge[47]. High-rated benefits are fisheries, timber, waste water treatment and coastal protection. An average estimate is 20,000 US$ per ha and per year, within a range of 500-100,000. This large range can (partly) be explained by differences in valuation methods and site-specific factors such as vegetation characteristics (e.g. species, age, tree density, height, root), sediments (e.g. depth, nutrients) and various environmental variables (e.g. location, salinity, debris). Restoration costs reported in the literature fall in an even wider range of US$ 500-400,000 per ha, which can be (partly) explained by the type of engineering interventions, planting vs. natural regeneration and labour costs. Comparing the figures for restoration (per ha) and for benefits (per ha and per year), in almost every case efficient restoration measures yield benefits that greatly exceed the costs[47].
Further reading
Spalding, M. 2011. World Atlas of Mangroves: Mark Spalding, Mami Kainuma and Lorna Collins (eds.) London, Washington D.C.: Earthscan 2010. ISBN 978-1-84407-657-4
Related articles
- Marine habitats and ecosystems
- Blue carbon revenues of nature-based coastal protection
- Characteristics of sedimentary shores
- Potential Impacts of Sea Level Rise on Mangroves
- Chenier
- Coastal mud belt
External links
- UN Mangrove Management
- http://www.ozcoasts.gov.au/indicators/mangrove_areas.jsp
- http://www.vliz.be/vmdcdata/mangroves/index.php
- http://www.glomis.com
- https://en.wikipedia.org/wiki/Mangrove
References
- ↑ 1.0 1.1 http://www.vliz.be/vmdcdata/mangroves
- ↑ 2.0 2.1 Karleskint G. 1998. Introduction to marine biology. Harcourt Brace College Publishers. p.378
- ↑ 3.0 3.1 Horstman, E.M., Dohmen-Janssen, C.M., Narra, P.M.F., van den Berg, N.J.F., Siemerink, M. and Hulscher, S.J.M.H. 2014. Wave attenuation in mangroves: A quantitative approach to field observations. Coastal Engineering 94: 47–62
- ↑ Bunting, P., Rosenqvist, A., Hilarides, L., Lucas, R.M., Thomas, T., Tadono, T., Worthington, T.A., Spalding, M., Murray, N.J. and Rebelo, L-M. 2022. Global Mangrove Extent Change 1996 – 2020: Global Mangrove Watch Version 3.0. Remote Sensing. https://zenodo.org/records/6894273
- ↑ Bertness M.D., Gaines, S.D. and Hay, M.E. 2001. Marine Community Ecology. Sinauer Associates, Inc. p. 550
- ↑ Pinet P.R. 1998.Invitation to Oceanography. Jones and Barlett Publishers. p. 508
- ↑ 7.0 7.1 7.2 Hogarth P.J. 1999. The biology of mangroves. Oxford University Press. p.228
- ↑ Saintilan, N., Wilson, N., Rogers, K., Rajkaran, A. and Krauss, K.W. 2014. Mangrove expansion and saltmarsh decline at mangrove poleward limits. Glob. Change Biol. 20: 147–57
- ↑ Parida, A. K. and Jha, B. 2010. Salt tolerance mechanisms in mangroves: A review. Trees 24: 199–217
- ↑ 10.0 10.1 10.2 http://en.wikipedia.org/wiki/Mangrove
- ↑ 11.0 11.1 11.2 11.3 Eric Coppejans - http://www.vliz.be/imis/imis.php?module=person&persid=134
- ↑ http://sofia.usgs.gov/virtual_tour/ecosystems/index.html
- ↑ Nguyen, L.T.M., Hoang, H.T., Choi, E. and Park, P.S. 2023. Distribution of mangroves with different aerial root morphologies at accretion and erosion sites in Ca Mau Province, Vietnam. Estuarine, Coastal and Shelf Science 287, 108324
- ↑ Syahid, L.N., Sakti, A.D., Ward, R., Rosleine, D., Windupranata, W. and Wikantika, K. 2023. Optimizing the spatial distribution of Southeast Asia mangrove restoration based on zonation, species and carbon projection schemes. Estuarine, Coastal and Shelf Science 293, 108477
- ↑ Du, Q., Qin,Z., Ming, S. and Zhang, C. 2021 Differences in the vertical accretion of sediment among mangrove species with different aerial root types. Estuarine, Coastal and Shelf Science 256, 107375
- ↑ 16.0 16.1 Krauss, K.W., McKee, K.L., Lovelock, C.E., Cahoon, D.R., Saintilan, N., Reef, R. and Chen, L. 2014. How mangrove forests adjust to rising sea level. New Phytologist 202: 19–34
- ↑ 17.0 17.1 Eric Coppejans – Course Biodiversity of aquatic food webs: from algae to marine mammals UGent
- ↑ http://www.fiddlercrab.info/u_lactea.html
- ↑ http://en.wikipedia.org/wiki/Snowy_Egret
- ↑ http://en.wikipedia.org/wiki/Agkistrodon_contortrix
- ↑ http://en.wikipedia.org/wiki/Diamondback_turtle
- ↑ 22.0 22.1 Worthington, T. and Spalding, M. 2018. Mangrove Restoration Potential: A global map highlighting a critical opportunity. Report, 26 October 2018. doi:10.17863/CAM.39153
- ↑ Menendez, P., Losada, I.J., Torres-Ortega, S., Narayan, S. and Beck, M.W. 2020. The global flood protection benefits of mangroves. Sci. Rep. 10, 4404
- ↑ Kelty, K., Tomiczek, T., Cox, D.T., Lomonaco, P. and Mitchell, W. 2022. Prototype-Scale Physical Model of Wave Attenuation Through a Mangrove Forest of Moderate Cross-Shore Thickness: LiDAR-Based Characterization and Reynolds Scaling for Engineering With Nature. Front. Mar. Sci. 8: 780946
- ↑ Chen, Q., Li, Y., Kelly, D.M., Zhang, K., Zachry, B. and Rhome, J. 2021. Improved modeling of the role of mangroves in storm surge attenuation. Estuarine, Coastal and Shelf Science 260, 107515
- ↑ Chaudhuri, P., Chaudhuri, S. and Ghosh, R. 2019. The Role of Mangroves in Coastal and Estuarine Sedimentary Accretion in Southeast Asia. Sedimentary Processes - Examples from Asia, Turkey and Nigeria. IntechOpen DOI: http://dx.doi.org/10.5772/intechopen.85591
- ↑ Krauss, K.W., Cahoon, D.R., Allen, J.A., Ewel, K.C., Lynch, J.C. and Cormier, N. 2010. Surface elevation change and susceptibility of different mangrove zones to sea-level rise on Pacific high islands of Micronesia Ecosystems 13: 129–143
- ↑ Kauffman, J.B., Adame, M.F., Arifanti, V.B., Schile-Beers, L.M., Bernardino, A.F.,Bhomia, R.K., Donato, D.C., Feller, I.C., Ferreira, T.O., Jesus Garcia, M.d.C., MacKenzie, R.A., Megonigal, J.P., Murdiyarso, D., Simpson, L. and Hernandez Trejo, H. 2020. Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 90, e01405
- ↑ Alongi, D.M. 2020. Global significance of mangrove blue carbon in climate change mitigation. Science 2020, 2, 57
- ↑ Wang, F., Sanders, C.J., Santos, I.R., Tang, J., Schurech, M., Kirwan, M.L., Kopp, R.E., Zhu, K., Li, X., Yuan, J., Liu, W. and Li, Z. 2021. Global blue carbon accumulation in tidal wetlands increases with climate change. National Science Review 8: nwaa296
- ↑ 31.0 31.1 Holguin, G., Vazquez, P. and Bashan, Y. 2001. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol. Fertil. Soils 33: 265–278
- ↑ 32.0 32.1 Romanacha, S.S., DeAngelis, D.L., Koh, H.L., Li, Y., Teh, S.Y., Barizan, R.S.R. and Zhai, L. 2018. Conservation and restoration of mangroves: Global status, perspectives, and prognosis. Ocean and Coastal Management 154: 72–82
- ↑ 33.0 33.1 Besset, M., Gratiot, N., Anthony, E.J., Bouchette, F., Goichot, M. and Marchesiello, P. 2019. Mangroves and shoreline erosion in the Mekong River delta. Estuarine. Coastal Shelf Sci. 226, 106263
- ↑ 34.0 34.1 34.2 Friess, D.A., Rogers, K., Lovelock, C.E., Krauss, K.W., Hamilton, S.E., Lee, S.Y., Lucas, R., Primavera, J., Rajkaran, A. and Shi, S. 2019. The state of the world’s mangrove forests: past, present and future. Annu. Rev. Environ. Resour. 44: 89–115
- ↑ Bozi, B.S., Figueiredo, B.L., Rodrigues, E., Cohen, M.C.L., Pessenda, L.C.R., Alves, E.E.N., de Souza, A.V., Bendassolli, J.A., Macario, K., Azevedo, P. and Culligan, N. 2021. Impacts of sea-level changes on mangroves from southeastern Brazil during the Holocene and Anthropocene using a multi-proxy approach. Geomorphology 390, 107860
- ↑ UNEP. 2006. In the frontline: shoreline protection and other ecosystem services from mangroves and coral reefs. p.36
- ↑ Winterwerp, J., Erftemeijer, P., Suryadiputra, N., Van Eijk, P. and Zhang, L. 2013. Defining eco-morphodynamic requirements for rehabilitating eroding mangrove-mud coasts. Wetlands 33: 515–526
- ↑ 38.0 38.1 Rotich, B., Mwangi, E. and Lawry, S. 2016. Where land meets the sea: a global review of the governance and tenure dimensions of coastal mangrove forests. Bogor, Indonesia: CIFOR; Washington, DC: USAID Tenure and Global Climate Change Program
- ↑ Phong, N.T. and Nuong, C.T. 2023. Thinning, selective harvesting and mangrove protection forests: Lessons learned and recommendations from the Vietnamese Mekong Delta. Estuarine, Coastal and Shelf Science 288, 108345
- ↑ Luom, T.T., Phong, N.T., Smithers, S. and Tai, V.T. 2021. Protected mangrove forests and aquaculture development for livelihoods. Ocean and Coastal Management 205, 105553
- ↑ 41.0 41.1 41.2 Lewis III, R.R. 2005. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24: 403–418
- ↑ Wodehouse, D.C. and Rayment, M.B. 2019. Mangrove area and propagule number planting targets produce sub-optimal rehabilitation and afforestation outcomes. Estuar. Coast. Shelf Sci. 222: 91–102
- ↑ 43.0 43.1 43.2 Winterwerp, J.C., Albers, T., Anthony, E.J., Friess, D.A., Mancheno, A.G., Moseley, K., Muhari, A., Naipal, S., Noordermeer, J., Oost, A., Saengsupavanich, C., Tas, S.A.J., Tonneijck, F.H., Wilms, T., Van Bijsterveldt, C., Van Eijk, P., Van Lavieren, E. and Van Wesenbeeck, B.K. 2020. Managing erosion of mangrove-mud coasts with permeable dams – lessons learned. Ecological Engineering 158, 106078
- ↑ Maza, M., Lara, J.L. and Losada, I.J. 2021. Predicting the evolution of coastal protection service with mangrove forest age. Coastal Engineering 168, 103922
- ↑ 45.0 45.1 Primavera, J.H., Savaris, J.P., Bajoyo, B.E., Coching, J.D., Curnick, D.J., Golbeque, R.L., Guzman, A.T., Henderin, J.Q., Joven, R.V., Loma, R.A. and Koldewey, H.J. 2012. Manual on Community-based Mangrove Rehabilitation, Mangrove Manual Series No.1 London. 240 pp.
- ↑ van Bijsterveldt, C.E.J., van der Wal, D., Mancheno, A.G., Fivash, G.S., Helmi, M. and Bouma, T.J. 2023. Can cheniers protect mangroves along eroding coastlines? – The effect of contrasting foreshore types on mangrove stability. Ecological Engineering 187, 106863
- ↑ 47.0 47.1 Su, J., Friess, D.A. and Gasparatos, A. 2021. A meta-analysis of the ecological and economic outcomes of mangrove restoration. Nature Communications 12: 5050
Please note that others may also have edited the contents of this article.
|