Difference between revisions of "1,2-Dichloroethane"
| Line 1: | Line 1: | ||
{{Definition|title=1,2-dichloroethane | {{Definition|title=1,2-dichloroethane | ||
| − | |definition=1,2-Dichloroethane is a clear chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is | + | |definition=1,2-Dichloroethane is a clear, chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is the formation of vinyl chloride, used in the production of a variety of plastic and vinyl products. These include important construction materials such as polyvinyl chloride (PVC) pipes, but also packaging materials, furniture, auto mobile parts, wall coverings and housewares. <ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp38.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 2001 TOXICOLOGICAL PROFILE FOR 1,2-DICHLOROETHANE]</ref>}} |
== Notes == | == Notes == | ||
Revision as of 11:30, 2 October 2009
Definition of 1,2-dichloroethane:
1,2-Dichloroethane is a clear, chemically manufactured liquid. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. The most common use of 1,2-dichloroethane is the formation of vinyl chloride, used in the production of a variety of plastic and vinyl products. These include important construction materials such as polyvinyl chloride (PVC) pipes, but also packaging materials, furniture, auto mobile parts, wall coverings and housewares. [1]
This is the common definition for 1,2-dichloroethane, other definitions can be discussed in the article
|
Notes
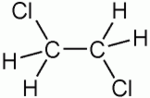
| 1,2-Dichloroethane |
|---|
|
| Formula |
| C2H4Cl2 |
US annual production of 1,2-dichloroethane averaged around 7 million tonnes in the 1990s. It can enter the environment during manufacture, transport or use. Most 1,2-dichloroethane is released to the air, although some is released to rivers or lakes. [1]
1,2-Dichloroethane can evaporate rapidly from water or soils to the atmosphere where it is slowly degraded. It can persist in the atmosphere, with a half-life of 5 months and can be transported over large distances. It has a moderate water solubility of 8,69 g/l and is not expected to adsorb to particles or sediments. In water it is also slowly degraded, almost not biodegraded and most removal takes place by evaporation. The half-life of 1,2-dichloroethane in water due to evaporation is 10 days. 1,2-Dichloroethane doesn't have a tendency to bioaccumulate and is therefore not expected to biomagnify through food chains.[1]
Concentrations of 1,2-dichloroethane which cause acute toxicity to marine fish are above 118 mg/l. the concentration which cause acute toxicity to fresh water fish, marine invertebrates and marine algae are above 30 mg/l, 36 mg/l and 100 mg/l respectively. [2]
It is suspected that 1,2-dichloroethane in the North Sea might reach concentrations up to 6,4 µg/l in heavily polluted coastal areas, concentrations in polluted estuaries typically range around 0,5 µg/l and those in open seas around 0.005 µg/l. [2]
Environmental standards and legislation
Included in the water framework list of priority substances