Difference between revisions of "Ethyl O-(p-nitrophenyl) phenyl phosphonothionate"
Dronkers J (talk | contribs) |
|||
| (5 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | {{Definition|title=ethyl O-(p-nitrophenyl) phenyl phosphonothionate ( | + | {{Definition|title=ethyl O-(p-nitrophenyl) phenyl phosphonothionate (EPN) |
|definition=Ethyl O-(p-nitrophenyl) phenyl phosphonothionate is an organic compound with a phosphorous group. It is used as a [[pesticide]]. At room temperature it appears as a yellow powder, but at temperatures above 35 °C it becomes a brown liquid.}} | |definition=Ethyl O-(p-nitrophenyl) phenyl phosphonothionate is an organic compound with a phosphorous group. It is used as a [[pesticide]]. At room temperature it appears as a yellow powder, but at temperatures above 35 °C it becomes a brown liquid.}} | ||
| Line 17: | Line 17: | ||
Ethyl O-(p-nitrophenyl) phenyl phosphonothionate has been used as an insecticide on a variety of plants against a wide range of insects. | Ethyl O-(p-nitrophenyl) phenyl phosphonothionate has been used as an insecticide on a variety of plants against a wide range of insects. | ||
| − | + | EPN is expected to be released released to the environment primarily during aerial and ground spraying of various agricultural crops. In water or the soil it is expected to be degraded rapidly (in less than a month) by hydrolysis and oxidation. Due to it's low water solubility it is expected to be relative immobile in the soils where it's sprayed upon. In water it is also mostly [[adsorption|adsorbed]] to suspended particles and sediments. It has a low [[volatile|volatility]], causing only small amounts to evaporate to the atmosphere where they are degraded rapidly<ref>[http://www.speclab.com/compound/c2104645.htm www.speclab.com August 12 2009]</ref>. | |
| − | <ref>[http://www.speclab.com/compound/c2104645.htm www.speclab.com]</ref> | ||
| − | EPN has some tendency to [[bioaccumulation|bioaccumulate]], but only when biota are continuously exposed, since EPN can be excreted rapidly | + | EPN has some tendency to [[bioaccumulation|bioaccumulate]], but only when biota are continuously exposed, since EPN can be excreted rapidly<ref>[http://www.find-health-articles.com/rec_pub_9297785-acute-toxicity-accumulation-excretion-organophosphorous-insecticides.htm Tsuda T, Kojima M, Harada H, Nakajima A, Aoki S; 1997 Acute toxicity, accumulation and excretion of organophosphorous insecticides and their oxidation products in killifish. Chemosphere. 35(5):939-49] </ref>. |
| + | |||
| + | It is a highly [[toxic]] compound for crustaceans; ENP concentrations above 0,5 µg/l can be lethal. [[Pollution and pelagic fishes|Fish]] are less vulnerable as concentrations up to 20 µg/l are tolerated, and some fish [[species]] can even survive short exposure to ENP concentrations above 1 mg/l<ref>[http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC35087 www.pesticideinfo.org August 12 2009]</ref>. | ||
| − | |||
| − | |||
<P> | <P> | ||
<BR> | <BR> | ||
| Line 37: | Line 36: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | {{author | ||
| + | |AuthorID=19826 | ||
| + | |AuthorFullName=Daphnis De Pooter | ||
| + | |AuthorName=Daphnisd}} | ||
| − | [[Category: | + | [[Category:Toxicity chemicals]] |
Latest revision as of 13:14, 9 August 2020
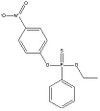
Definition of ethyl O-(p-nitrophenyl) phenyl phosphonothionate (EPN):
Ethyl O-(p-nitrophenyl) phenyl phosphonothionate is an organic compound with a phosphorous group. It is used as a pesticide. At room temperature it appears as a yellow powder, but at temperatures above 35 °C it becomes a brown liquid.
This is the common definition for ethyl O-(p-nitrophenyl) phenyl phosphonothionate (EPN), other definitions can be discussed in the article
|
Notes
| EPN |
|---|
|
| Formula |
| C14H14NO4PS |
Ethyl O-(p-nitrophenyl) phenyl phosphonothionate has been used as an insecticide on a variety of plants against a wide range of insects. EPN is expected to be released released to the environment primarily during aerial and ground spraying of various agricultural crops. In water or the soil it is expected to be degraded rapidly (in less than a month) by hydrolysis and oxidation. Due to it's low water solubility it is expected to be relative immobile in the soils where it's sprayed upon. In water it is also mostly adsorbed to suspended particles and sediments. It has a low volatility, causing only small amounts to evaporate to the atmosphere where they are degraded rapidly[1].
EPN has some tendency to bioaccumulate, but only when biota are continuously exposed, since EPN can be excreted rapidly[2].
It is a highly toxic compound for crustaceans; ENP concentrations above 0,5 µg/l can be lethal. Fish are less vulnerable as concentrations up to 20 µg/l are tolerated, and some fish species can even survive short exposure to ENP concentrations above 1 mg/l[3].
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
References
Please note that others may also have edited the contents of this article.
|