Difference between revisions of "EDTA"
(ref) |
(ref) |
||
| Line 1: | Line 1: | ||
| − | |||
| − | EDTA acid was patented in Germany in 1935 | + | {{Definition|title= ethylenediaminetetraacetic acid (EDTA) |
| + | |||
| + | |definition= EDTA acid was patented in Germany in 1935. The molecule is a substituted diamine usually marketed as its sodium salts. At room temperature it occurs as a white powder. It is a powerful complexing agent of metals and a highly stable molecule, offering a considerable versatility in industrial and household uses2. Since it is applied predominantly in aqueous medium, it is released into the environment through wastewaters.<ref name="clau">[http://www.scielo.br/pdf/qn/v26n6/a20v26n6.pdf Claudia Oviedo and Jaime Rodríguez 2003 EDTA: THE CHELATING AGENT UNDER ENVIRONMENTAL SCRUTINY Quim. Nova, Vol. 26, No. 6, 901-905, 2003]</ref>}} | ||
== Notes == | == Notes == | ||
| − | EDTA is mainly used in detergents and in the pulp and paper industry. Its use in Europe has increased in recent years from 26.000 tons in 1992 to 32.550 tons in 1997. Yearly 266 tonnes end of in river waste waters. | + | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" |
| + | ! bgcolor="#FF8888" | EDTA | ||
| + | |- | ||
| + | | align="center" bgcolor="#FFFFFF" | [[Image:EDTA.png|200px|EDTA]] | ||
| + | |- | ||
| + | ! bgcolor="#8888FF" | Formula | ||
| + | |- | ||
| + | | align="center" | C<sub>10</sub>H<sub>16</sub>N<sub>2</sub>O<sub>8</sub> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | EDTA is mainly used in detergents and in the pulp and paper industry. Its use in Europe has increased in recent years from 26.000 tons in 1992 to 32.550 tons in 1997. Yearly 266 tonnes end of in river waste waters. <ref name="eu">[http://www.baua.de/nn_8874/de/Chemikaliengesetz-Biozidverfahren/Dokumente/RAR__062.pdf European Union 2004 Risk Assessment Report tetrasodium ethylenediaminetetraacetate(Na4EDTA)] </ref> | ||
In natural environments EDTA occurs as metal–EDTA complexes. It is very stable molecule, which is not very biodegradable. In fact in surface waters, the only significant process of removal of EDTA is the possibility of photolysis by sunlight. In water it has a high solubility of 500 g/l and is expected not to [[adsorption|adsorb]] to particles or sediments. It doesn't [[bioaccumulation|bioaccumulate]] in any organism. | In natural environments EDTA occurs as metal–EDTA complexes. It is very stable molecule, which is not very biodegradable. In fact in surface waters, the only significant process of removal of EDTA is the possibility of photolysis by sunlight. In water it has a high solubility of 500 g/l and is expected not to [[adsorption|adsorb]] to particles or sediments. It doesn't [[bioaccumulation|bioaccumulate]] in any organism. | ||
EDTA however does bind [[heavy metals|metals]] and forms complexes with them. By doing so it enhances their mobility and bioavailability. EDTA can avoid the precipitation of heavy metals in solution and it can even cause heavy metals adsorbed in sediments to dissolve. | EDTA however does bind [[heavy metals|metals]] and forms complexes with them. By doing so it enhances their mobility and bioavailability. EDTA can avoid the precipitation of heavy metals in solution and it can even cause heavy metals adsorbed in sediments to dissolve. | ||
| − | The metal-EDTA complexes are assumed to be less toxic than the free metals themselves, except for [[ | + | The metal-EDTA complexes are assumed to be less toxic than the free metals themselves, except for [[cadmium]] and [[copper]] which might be more toxic when bound to EDTA. |
| + | <ref name="clau">[http://www.scielo.br/pdf/qn/v26n6/a20v26n6.pdf Claudia Oviedo and Jaime Rodríguez 2003 EDTA: THE CHELATING AGENT UNDER ENVIRONMENTAL SCRUTINY Quim. Nova, Vol. 26, No. 6, 901-905, 2003]</ref> | ||
| + | |||
| + | Acute toxicity can occur in fishes and algae at concentrations above 50 mg/l. | ||
| + | Concentrations in fresh surface waters are expected to be around 26 µg/l.<ref name="eu">[http://www.baua.de/nn_8874/de/Chemikaliengesetz-Biozidverfahren/Dokumente/RAR__062.pdf European Union 2004 Risk Assessment Report tetrasodium ethylenediaminetetraacetate(Na4EDTA)] </ref> | ||
| − | + | <P> | |
| − | + | <BR> | |
| − | + | <P> | |
| + | == Environmental standards and legislation == | ||
| − | + | [[List of priority substances|Included in the water framework list of priority substances]] | |
| − | + | <P> | |
| − | + | <BR> | |
| − | + | <P> | |
| − | + | ==References== | |
| − | < | + | <references/> |
| + | |||
| + | [[Category:Coastal and marine pollution]] | ||
Revision as of 12:55, 24 August 2009
Definition of ethylenediaminetetraacetic acid (EDTA):
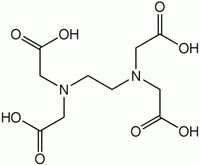
EDTA acid was patented in Germany in 1935. The molecule is a substituted diamine usually marketed as its sodium salts. At room temperature it occurs as a white powder. It is a powerful complexing agent of metals and a highly stable molecule, offering a considerable versatility in industrial and household uses2. Since it is applied predominantly in aqueous medium, it is released into the environment through wastewaters.[1]
This is the common definition for ethylenediaminetetraacetic acid (EDTA), other definitions can be discussed in the article
|
Notes
| EDTA |
|---|
|
| Formula |
| C10H16N2O8 |
EDTA is mainly used in detergents and in the pulp and paper industry. Its use in Europe has increased in recent years from 26.000 tons in 1992 to 32.550 tons in 1997. Yearly 266 tonnes end of in river waste waters. [2]
In natural environments EDTA occurs as metal–EDTA complexes. It is very stable molecule, which is not very biodegradable. In fact in surface waters, the only significant process of removal of EDTA is the possibility of photolysis by sunlight. In water it has a high solubility of 500 g/l and is expected not to adsorb to particles or sediments. It doesn't bioaccumulate in any organism. EDTA however does bind metals and forms complexes with them. By doing so it enhances their mobility and bioavailability. EDTA can avoid the precipitation of heavy metals in solution and it can even cause heavy metals adsorbed in sediments to dissolve. The metal-EDTA complexes are assumed to be less toxic than the free metals themselves, except for cadmium and copper which might be more toxic when bound to EDTA. [1]
Acute toxicity can occur in fishes and algae at concentrations above 50 mg/l.
Concentrations in fresh surface waters are expected to be around 26 µg/l.[2]
Environmental standards and legislation
Included in the water framework list of priority substances