Difference between revisions of "Hexachlorobutadiene"
| Line 1: | Line 1: | ||
{{Definition|title= hexachlorobutadiene (HCBD) | {{Definition|title= hexachlorobutadiene (HCBD) | ||
| − | |definition= Hexachlorobutadiene, also known as perchlorobutadiene is a colourless liquid and has a turpentine-like odour. <ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp42.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 1994 TOXICOLOGICAL PROFILE FOR HEXACHLOROBUTADIENE]</ref>}} | + | |definition= Hexachlorobutadiene, also known as perchlorobutadiene, is a colourless liquid and has a turpentine-like odour. <ref name="US">[http://www.atsdr.cdc.gov/toxprofiles/tp42.pdf U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES 1994 TOXICOLOGICAL PROFILE FOR HEXACHLOROBUTADIENE]</ref>}} |
===Notes=== | ===Notes=== | ||
Revision as of 11:21, 28 August 2009
Definition of hexachlorobutadiene (HCBD):
Hexachlorobutadiene, also known as perchlorobutadiene, is a colourless liquid and has a turpentine-like odour. [1]
This is the common definition for hexachlorobutadiene (HCBD), other definitions can be discussed in the article
|
Notes
| Hexachlorobutadiene |
|---|
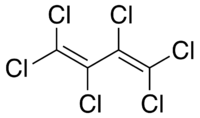
|
| Formula |
| C4Cl6 |
Historically HCBD was used as a solvent for rubber and other polymers, heat transfer fluids, transformer liquid, hydraulic fluid and washing liquor for removing hydrocarbons. It has also been used in agriculture as a seed dressing and fungicide for a variety of crops and has found applications in a number of manufacturing processes such as production of aluminium and graphite rods. Due to environmental concerns the use of HCBD in such applications has now virtually ceased, although it is possible that HCBD may still be in use in some parts of the world. HCBD is however still generated as a by-product of tetrachlorethene and tetrachloromethane production. HCBD mainly enters the environment during processing: in 1997 EU countries emitted 2 kg of HCBD into the atmosphere and 100 kg into the hydrosphere. [2]
Hexachlorobutadiene has quite a high vapour pressure and is therefore mainly present into the atmosphere (98%). In the atmosphere half its concentration may be broken down in 60 days although some studies suggested this might take more than a year. In water it has a low solubility of 3,2 mg/l and has a tendency to adsorbe to suspended particles and sediments. In fact only 0,2 percent of the environmental HCBD is expected to be dissolved in water and the other 1,8% adsorbed to sediments and soils. In aerobic waters and soils, HCBD can be biodegraded, although it may take between 30 and 300 days to half its concentration in water. [1]
Hexachlorobutadiene has a high potential to bioaccumulate. High bioaccumulation rates have been shown in a number of marine species, although in others hardly any bioaccumulation took place. Therefore some species appear to be more able to metabolise HCBD than others. [1] Fish seem to accumulate high levels in their livers, about 10 times more than in other tissues. HCBD seems to have a lower tendency to bioaccumulate through food uptake than through direct uptake from water. One study which fed predatory fish with contaminated prey fish in fact demonstrated lower concentrations in the predatory fish. A study where rats were fed with contaminated food also didn't demonstrate any bioaccumulation. Therefore the risks of secondary poisoning for marine mammals and sea birds by biomagnification are doubtful.[2]
Acute toxicity in marine fishes can be caused by concentrations above 0,45 mg/l, in algae by concentrations above 0,85 mg/l. Birds fed during 90 days with food containing 10 mg/kg HCBD displayed a reduced fertility, caused by a reduced chick survival. [2]
Measured marine surface water concentrations of HCBD in Europe have reached values up to 150 ng/l in polluted estuaries, although typical North Sea concentrations are around 4 ng/l. The highest concentration measured in fish tissue was 0,4 µg/kg. [2]
Environmental standards and legislation
Included in the water framework list of priority substances