Difference between revisions of "PAH"
(n) |
|||
| Line 17: | Line 17: | ||
|} | |} | ||
| − | PAHs have been become increasingly important in research because they are potentially mutagenic or carcinogenic in aquatic organisms and man. They tend to be [[adsorption|adsorbed]] and [[bioaccumulation|accumulated]] by marine organisms. Elevated levels of PAHs are commonly found in coastal and [[estuary|estuarine]] marine waters near heavily polluted areas. Not all PAHs are known to be carcinogenic, but the environmental effect of most of them remains uncertain, and little is known about how they behave in marine ecosystems. | + | PAHs have been become increasingly important in research because they are potentially mutagenic or carcinogenic in aquatic organisms and man. They tend to be [[adsorption|adsorbed]] and [[bioaccumulation|accumulated]] by marine organisms. Elevated levels of PAHs are commonly found in [[coastal area|coastal]] and [[estuary|estuarine]] marine waters near heavily [[pollution|polluted]] areas. Not all PAHs are known to be carcinogenic, but the environmental effect of most of them remains uncertain, and little is known about how they behave in marine ecosystems. |
They tend to adsorb to particles, as these particles sink to the bottom, the sea floor becomes enriched in PAH content. Since PAHs are quite stable in the sediments, bottom dwelling organisms can be exposed by large amounts of PAHs for long periods of time. | They tend to adsorb to particles, as these particles sink to the bottom, the sea floor becomes enriched in PAH content. Since PAHs are quite stable in the sediments, bottom dwelling organisms can be exposed by large amounts of PAHs for long periods of time. | ||
| − | Some species (like fish) can excrete PAH rather fast, therefore they have lower body burdens of PAHs than for example shellfish who | + | Some [[species]] (like [[pollution and benthic fishes|fish]]) can excrete PAH rather fast, therefore they have lower body burdens of PAHs than for example shellfish who bioaccumulate PAHs. The most toxic PAHs are pyrene and [[fluoranthene]], followed by phenanthene and [[anthracene]]. PAHs with a high molecular weight may cause sublethal effects; such as growth reduction, chronic diseases, reproductive impairment, at very low concentrations in [[biota]]: at 5 to 100 ppb (parts per billion) in the tissue of the animal. While concentrations which are considered toxic range from 0,2 to 10 ppm (parts per million). Concentrations in wild biota inside this range have been found in heavily polluted areas<ref>Biology of marine birds. Schreiber, E.A. & Burger, J. (Eds). 2002. Boca Raton, Florida: CRC Press. 722 pp. </ref> <ref>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref>. |
| − | <ref>Biology of marine birds. Schreiber, E.A. & Burger, J. (Eds). 2002. Boca Raton, Florida: CRC Press. 722 pp. </ref> | ||
| − | <ref>Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp</ref> | ||
| − | |||
== Case studies == | == Case studies == | ||
Revision as of 09:39, 31 August 2009
Definition of polycyclic aromatic hydrocarbon (PAH):
Polycyclic aromatic hydrocarbons are any of several multi-ringed aromatic compounds and are found in e.g. soot, coal, tar, cigarette smoke and barbecue meat. Examples include pyrene and benzo(a)-pyrene. Some of these compounds are known carcinogens.
[1]
This is the common definition for polycyclic aromatic hydrocarbon (PAH), other definitions can be discussed in the article
|
Notes
| Pyrene |
|---|
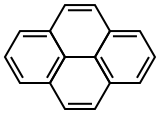
|
| Formula |
| C16H10 |
PAHs have been become increasingly important in research because they are potentially mutagenic or carcinogenic in aquatic organisms and man. They tend to be adsorbed and accumulated by marine organisms. Elevated levels of PAHs are commonly found in coastal and estuarine marine waters near heavily polluted areas. Not all PAHs are known to be carcinogenic, but the environmental effect of most of them remains uncertain, and little is known about how they behave in marine ecosystems.
They tend to adsorb to particles, as these particles sink to the bottom, the sea floor becomes enriched in PAH content. Since PAHs are quite stable in the sediments, bottom dwelling organisms can be exposed by large amounts of PAHs for long periods of time.
Some species (like fish) can excrete PAH rather fast, therefore they have lower body burdens of PAHs than for example shellfish who bioaccumulate PAHs. The most toxic PAHs are pyrene and fluoranthene, followed by phenanthene and anthracene. PAHs with a high molecular weight may cause sublethal effects; such as growth reduction, chronic diseases, reproductive impairment, at very low concentrations in biota: at 5 to 100 ppb (parts per billion) in the tissue of the animal. While concentrations which are considered toxic range from 0,2 to 10 ppm (parts per million). Concentrations in wild biota inside this range have been found in heavily polluted areas[2] [3].
Case studies
PCB and heavy metals in beached sperm whales
Environmental standards and legislation
Included in the OSPAR list of substances of priority action
Included in the water framework list of priority substances
See also
OSPAR background document on PAH
References
- ↑ Lawrence E (ed.), 2000. Henderson’s Dictionary of Biological Terms. 12th edition. Prentice Hall, Pearson Education Limited. Harlow, Great Britain.
- ↑ Biology of marine birds. Schreiber, E.A. & Burger, J. (Eds). 2002. Boca Raton, Florida: CRC Press. 722 pp.
- ↑ Kennish, M. J. (1996): Practical Handbook of Estuarine and Marine Pollution, CRC Press 524 pp