Meiofauna of Sandy Beaches
The surface of sandy beaches is permanently moving under the action of the tides, wind and currents. It is a reason why a sandy shoreline provides no place for attachment of surface-growing seaweeds and no inviting crevices, but it can hold water between small grains. Beneath the water surface, the environment is unaffected by the weather. Sandy beaches appear dead and inhospitable, like a desert. However, a wide variety of organisms inhabit the space between the sediment particles, in damp sand, on sandy shore, among them most abundant – meiofauna communities.
Contents
Meiofauna
The term „Meiofauna“ is related to microscopically small benthic invertebrates that live in both marine and fresh water environments. Meiofauna formally defined as a group of organisms by their size, larger than microfauna but smaller than macrofauna. In practice these are metazoan (some researchers include protozoan as well) animals that can pass unharmed through a 0.5 – 1 mm mesh but will be retained by a 30 μm mesh but the exact dimensions will vary from researcher to researcher. Nowadays the term meiofauna is used interchangeably with meiobenthos. Meiofauna is mainly found in and on soft sediments, but also on underwater algae and higher plants as well as on other hard substrates. The heterogeneity of meiofaunal habitats is so large and meiobenthic taxa so diverse.
Interstitial Biota
Practically most classes of the Metazoa are represented in the meiofauna of sandy beaches, while the Protozoa are represented by their largest forms, e.g., Foraminifera and Ciliata. The smaller protozoans are generally considered as microfauna.
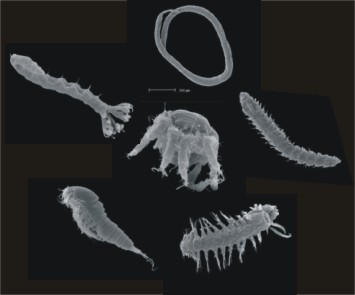
On most beaches, the interstitial fauna is rich and diverse, even exceeding the macrofauna biomass. There may be as many as 25 meiofauna species for every macrofauna species (Armonies and Reise 2000). The reson is simple – greater stability and complexity of the interstitial habitat.Most common metazoan phyla represented in the benthic meiofauna (Figure 1):
- Cnidaria - Hydroida, Scyphozoa, Anthozoa
- Platyhelminthes – Turbellaria
- Gnathifera – Gnathostomulida, Rotifera, Rotatoria
- Nemertinea
- Nemathelminthes – Nematoda, Kinorhyncha, Priapulida, Loricifera, Gastrotricha
- Tardigrada
- Crustacea – Cephalocarida, Ostracoda, Mystacocarida, Copepoda (e.g. Harpacticoida, Cyclopoida), Malacostraca (e. g. Tanaidacea, Isopoda, Amphipoda)
- Chelicerata – Acari
- Annelida – Polychaeta, Oligochaeta
- Sipuncula
- Mollusca – Aplacophora, Gastropoda
- Tentaculata – Bryozoa
- Echinodermata - -Holothuroidea
- Chaetognatha
- Tunicata – Ascidiacea
This list of meiofauna taxa is far from being complete and have to be supplemented in the future, particulate since recent investigations of high-ranking taxa have brought meiofauna into the mainstream of invertebrate phylogeny (Giere 2009).
Meiofauna is the best-studied component of the interstitial biota. These small creatures are considered as temporary meiofauna if they are larval stages of macrofauna and permanent meiofauna if their entire life cycle is spent in previous mentioned size category (McLachlan & Brown 2006). McIntyre (1969) used the term permanent members (or permanent meiofauna) for species belonging to the meiofauna during the whole of their life cycle in marine systems in contrast to temporary meiofauna. The dominant taxa of sandy beach meiofauna are nematodes and harpacticoid copepods, with other important group including turbelarians, oligochaetes, ostracods or gastrotrichs.
Sampling and Processing Sandy Meiofauna
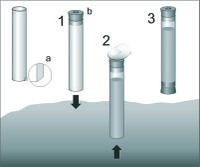
Meiofauna is usually collected from the sediment cores, obtained from a perplex tubes of 3,6 cm diameter (surface ~10 cm-2 is appropriate for all types of sediment), inserted 15 cm deep into the seabed (Figure 2). From one site six (at least three) replicates are taken. Sediment is gently pushed out from the tube and cut into the slices. Te slices are usually taken from particular layers: 0-1, 1-2, 2-3, 3-4, 4-5, 5-10 and 10-15 cm. Sometimes core is cut every 1 cm into even slices. Each sediment slice (subsample) is placed into the separate jar, fixed with 4 % neutral formaldehyde solution and stained with Bengal Rose, to obtain a pink/reddish color of the sample. For extraction of meiofauna from sediment are used two methods depending of the amounts of the detritus or silt-clay in the sediment. Decantation method - when the sediment is a sand with low amounts of detritus or silt-clay. In the laboratory, each slice is placed in the 1000 ml cylinder, filled with tap water and shaken vigorously, to suspend the sediment grains. The water is then filtered through 0.030 mm screen, and the procedure of shaking and flotation is repeated 10 times. All meiofauna organisms are retained on the screen, are gently washed into the Petri dish with measuring grid on the bottom and counted under the low power stereo microscope. Density gradient centrifugation method - the extraction from mud or detritus is most efficiently using a density gradient in a centrifugation procedure (Heip et al. 1985). In this method liquid with a density larger than the density of meiofaunal organisms can be used (Ludox, density of 1.15) The method consist: placement of sediment sample into the Ludox solution, centrifuge it with 1800 g for 10 min. The meiofauna organisms are retained in the 'gel', once the sediment is on the end of the tube. Repeat centrifugation three times more. This method does not work for heavy foraminifera, since their density is often equal to the sediment grain. In the lab, the meiofauna organisms are counted on the measuring grid and identified.Adaptations to Sandy Biotope
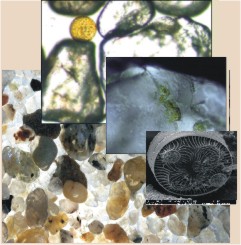
If we refrain from an exact division according to type of biotope but are satisfied to compare the three main groups—mud, interstitial sand, and phytal meiofauna — we find certain morphological and biological features for each of these categories, reflecting the adaptation of the organisms to particular environmental conditions. These adaptations undoubtedly are most marked in the interstitial fauna of sand (Figure 3).Sediment consist of sand particles which are mostly round due to abrasion. Usually spaces between them occupy 30-40% of the sediment volume. It creates a system of interstices which is filed with water, air, detritus and organisms. The smallest animals living in the sediment, the interstitial communities, move through the sediment, using the film of water which surrounds individual particles of sand. Interstitial animals therefore display numerous adaptations to resist one of the harshest environment for life on the surface of the planet. Organisms living here must be able to withstand the marine conditions of inundation by salt water which alternates with exposure to terrestrial conditions. Common sand meiofaunal adaptations by Giere (2009):
- to narrow spaces – miniaturization, elongation and flexibility
- to the mobile environment – adhesion, special locomotion and reinforcing structures
- to the three-dimensional dark conditions – static organs, reduction of pigments and eyes
Additionally interstitial organisms show specific adaptation related to reproduction and development – the production of only few eggs, direct sperm transfer or internal fertilization, brood protection, abbreviated larval life and restricted propagation (Giere 2009).
Meiofauna Abundance and Distribution
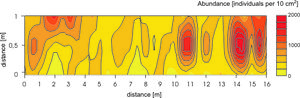
Large numbers of microscopic organisms occupy the system of interstitial canals. This porous system averages 40% of the total sediment volume. On the average abundance of metazoans organisms amount to 106 – 108 individuals per square meter.These values vary according to season, latitude, exposure, grain size etc. Populations of sandy beach animals are not randomly distributed, of course. The majority of the meiofauna has been found in the upper 2 cm layer. And this vertical zonation is mainly controlled by redox potential discontinuity level. Across sandy habitats species are also often restricted to certain zones – horizontal zonation. Additionally it is well known meiofauna is patchy distributed, even in very small scale, but causes of such distributions are still difficult to define (Figure 4).
Role of Meiofauna
Meiofauna plays an important role as a trophic link between bacteria and larger fauna. It enhances the rate of carbon mineralisation by stimulating microbial activity through predation, and/or consumption of detritus by larger deposit-feeding invertebrates.In sediments meiofauna:
- facilitate biomineralization of organic matter and enhance nutrient regeneration
- serve as food for a variety of higher trophic levels
- exhibit high sensitivity to anthropogenic inputs, making them excellent sentinels of pollution.
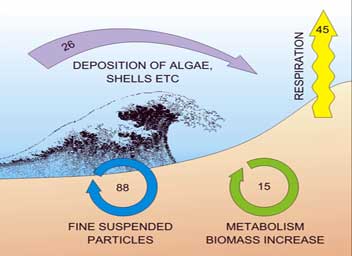
Generally mineralization of organic matter is enhanced and bacterial production stimulated in the presence of meiofauna (Figure 5). Meiofauna, particularly copepods, are known foods for a variety of predators especially juvenile fish and the meiofaunal copepods are high in the essential fatty acids required by fish. Most contaminants reside in sediments, and meiofauna are intimately associated with sediments over their entire life-cycle, thus they are increasingly being used as pollution sentinels. Because meiofauna have short lifecycles, the effects of a contaminant on the entire life-history can be assessed within a relatively short time. The use of modern molecular biology techniques to assess genetic diversity of meiofauna in contaminated vs uncontaminated sediments is a promising avenue for future research.
The marine meiofauna, defined as animals of microscopic size living in marine sediments, is one the earth’s richest and most diverse community extending from the shore to the deep sea. The marine meiofauna still contains numerous undescribed species and higher taxa. Special morphological adaptations evolved, especially in meiofauna living in the intertidal zone which is under a strong abiotic regime. Certain higher taxa evolved exclusively in the marine interstitial system. Evolutionary constrains caused elaborated life-cycles, migration patterns, special reproductive behaviours and structural adaptations. The interstitial system is also habitat for larvae and juveniles of certain macrofaunal species. A surprisingly large number of species coexists in the tiny interstices, but still most questions on their interactions and life strategies await their answers.
Please note that others may also have edited the contents of this article.
|