Difference between revisions of "Oxygen sensors"
Cliviahaese (talk | contribs) (→Electrochemical (Clark Electrode)) |
|||
| Line 103: | Line 103: | ||
<references/> | <references/> | ||
| − | {{ | + | {{2Authors |
|AuthorID1=5068 | |AuthorID1=5068 | ||
|AuthorName1=Wikischro | |AuthorName1=Wikischro | ||
Revision as of 14:47, 3 August 2011
Back to Instruments and sensors to measure environmental parameters
In this article two different types of sensors are described, the electrochemical and the optical type of oxygen sensor.
Contents
Oxygen Sensor: Electrochemical (Clark Electrode)
Physical Relationships in seawater
Oxygen exists in a dissolved state in seawater. A state of equilibrium is reached when the partial pressure of oxygen, i.e. the part of the total pressure that is due to oxygen, is equal in air and in seawater. The seawater is then saturated with oxygen. In dry, atmospheric air, the oxygen partial pressure (20.95% of the air pressure) is reduced over a water surface because water vapour has its own vapour pressure and a corresponding partial pressure.
When air is saturated the partial pressure of oxygen pO2 is PO2 =0.2095 (Pair –Pvapour) Equ. 1 with Pair= air pressure and Pvapour=water vapour pressure PO2 and Pvapour are temperature-dependent
The vapour pressure of water calculated from an empirical equation derived from the Handbook of Chemistry and Physics (Chemical Rubber Company, (Cleveland, Ohio, 1964) is log Pvapour = 8.10765 (1750.286/235+t)) (3) where t is temperature in degrees C.
The oxygen concentration cO2 in water is cO2=(aO2 PO2 MO2) / VM with aO2 = Bunsen coefficient, MO2= molar mass of oxygen and VM= molar volume. cO2, PO2 and aO2 are temperature-dependent (Remark: The Bunsen coefficient (aO2) is defined as the volume of oxygen, reduced to 0°C and 1 atm (101.325 kPA), which is absorbed by the unit volume of seawater at the temperature of measurement when the partial pressure of oxygen is equal to one standard atmosphere).
Due to the different temperature-dependences an exact knowledge of the temperature is important. Since water vapour pressure increases as temperature rises, the partial pressure of oxygen decreases. The salt content of seawater decreases the solubility of oxygen in comparison to fresh water. Therefore, the oxygen concentration depends on salinity.
For the assessment of biological processes under varying salinity (estuary, coastal waters) and temperature very often the saturation index is used: The oxygen saturation is calculated as the percent of dissolved oxygen relative to a theoretical maximum concentration given the temperature, pressure, and salinity of the water. The oxygen saturation index shows the over- or under-saturation of a water body due to biological processes -independent of salinity or temperature. For a calculation of the oxygen saturation at a specific temperature and salinity corrections from the US Geological survey can be found at http://water.usgs.gov/admin/memo/QW/qw81.11.html
The calculations are based on an equation by Weiss (1970, Deep-Sea Res. 17:721-735) which fits the data by Carpenter (1966, Limnol. Oceanogr. 11:264-277) with a maximum difference of -0.04 mg/L. Carpenter's values are, at the present time, widely accepted as the most accurate determinations of saturation DO available. Tables for salinity correction can be found at http://water.usgs.gov/owq/FieldManual/Chapter6/6.2.4.pdf
Description of the Sensor
The basic principle underlying the electrochemical determination of oxygen concentration is the use of membrane-covered polarographic sensors. The main components of the sensors are the oxygen-permeable membrane (mainly Teflon), the working electrode, the counter electrode, the electrolyte solution and a reference electrode. A voltage is applied between the gold cathode and the silver anode and causes the oxygen to react electrochemically. The higher the oxygen concentration, the higher the resulting electric current will be. The current in the sensor is measured and, after calibration, converted into the concentration of dissolved oxygen.
During this process the cathode provides electrons and the oxygen that diffuses through the membrane reacts with water to form hydroxide ions. The metal of the electrode is oxidized at the anode, a process that releases the electrons required for the cathode reaction. The components of the electrolyte solution bind the metal ions generated by the anode reaction.
With the addition of another silver/silver bromide electrode, the polarographic sensors can be connected to form a three-electrode cell. They no longer have an anode in the classical sense. One of the silver/silver bromide electrodes acts as a counter electrode (to provide the current) and the other one acts as an independent reference electrode. Current does not flow through this electrode and, thus, it can maintain a much more constant potential than a conventional electrode could.
In the preceding chapter the significance of the sample temperature for oxygen measurements was already outlined (dependency of the various variables on temperature). Furthermore, the oxygen permeability of the membrane is temperature-dependents. Therefore, in addition to the external temperature probe (sample temperature!), another probe is required and is built into the sensor head. With these two temperature values, the instrument can compensate for the influence of temperature on the oxygen permeability of the membrane.
Another important factor for in situ oxygen probes is the flow of water across the membrane that has to be ensured during measurement in order to prevent an oxygen-depletion near the membrane.
The membrane properties mainly determine the stability of the sensor: Since inside the sensor chamber oxygen is consumed by the polarographic process there is a steep gradient of the oxygen concentration over the membrane. This gradient is dependent on the thickness and permeability of the membrane. Any changes, e.g., an increased permeability due to a biofilm formation on the membrane (biofouling), directly changes the reading of the sensor. Another critical factor for these sensors is the mechanical behaviour of the membrane: If the tension changes due to handling or stress there is a direct influence on the measured value. An example is an Endreß & Hauser oxygen sensor COS 4 with electronic unit LiquiSys COM 223 (Fig. 1) The measuring range of the sensor is 0.05- 20 mg/L; the time constant is 3 min (90%).
Calibration of the Sensor
Because the measuring process consumes the electrolyte solution in the sensor head calibration must also be carried out for dissolved oxygen measurements at regular intervals. The ions of the electrolyte solution bind the released metal ions, thereby changing the composition of the solution. The recommended calibration interval depends on the oxygen sensor used and ranges from two weeks to one month. In principle, each linear calibration function is defined by two points: Zero and near 100% saturation. However, with modern sensors such as the Endress & Hauser sensor, the sensor signal obtained in the absence of oxygen (“zero point”) lies below the resolution of the sensor. Therefore, only a one-point calibration has to be carried out.
There are three possibilities for calibration of oxygen sensors
- Comparison with water samples and measurement by Winkler titration.
This is the most exact method, however the whole process is complex and requires experienced personal.
- Calibration in air-saturated water.
This method is prone to errors concerning over-saturation of the water under certain circumstances. Care has to be taken to provide enough water movement (stirring, bubbling of air) during the calibration.
- Calibration in water-saturated air.
When using this method care has to be taken to avoid temperature fluctuations by evaporation etc. in the closed calibration chamber. The calibration is carried out by placing the sensor into a small semi-closed container with a wet sponge at the bottom (free pressure exchange with the atmosphere is necessary). It is important to ensure that there are no water droplets on the membrane. Otherwise, the calibration would partially take place in water! It is particularly important to take precautions after the sensor has been stored in the calibration vessel for an extended period of time and condensation droplets may have formed on the membrane.
Modern instruments such as the one by Endress & Hauser (Fig. 1) totally automate the calibration process: After having the sensor equilibrated in the enclosed container just the appropriate method (“water in air”) is chosen. All necessary constants are stored in the instrument and the slope is determined automatically.
Reliability and Applicability
The Endress & Hauser oxygen electrode is a robust sensor. It is very stable due to its large membrane-covered area and relatively thick membrane (which increases the time constant to about 3 min).
Under normal conditions, a membrane lasts for more than a year before it has to be replaced (together with an replacement of the internal electrolyte). At this occasion a cleaning/regeneration of the silver anode should be carried out. Cleaning can be automated, as for example in a FerryBox system with acidified water. Then, a calibration only had to be carried out in intervals of 1-2 months. However, this not necessarily can be applied on other systems with different cleaning procedures.
Oxygen Sensor: Fluorescence quenching ("Optode")
Description of the Sensor
Tenberg et al (submitted) have produced a paper, which describes in detail the development of the idea of the oxygen optode and its application in a number of environments including long-term deployments on mooring. The reference point for all oxygen measurements is the “Winkler Method”. The standard method to determine concentrations of oxygen in water is a two-step wet chemistry precipitation of the dissolved oxygen followed by a titration. The method was first described by Winkler (1888) and has since become the standard method. Winkler titration is always performed on collected water samples. The collection and handling of water samples can induce errors and the analytical work is time consuming and demands meticulous care. It is therefore not a suitable method to obtain in-situ data with high spatial and temporal resolution.
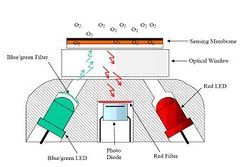
Tenberg et al (submitted) have shown that the oxygen optode provides a more suitable method than electrochemical sensors for direct measurement of dissolved oxygen. Optode technology has been known for years but it is relatively new to the aquatic research. The fundamental principle is based on the ability of selected substances to act as dynamic fluorescence quenchers. In the case of oxygen, if a ruthenium complex is illuminated with a blue light it will be excited and emits a red luminescence with an intensity and lifetime that depends on the ambient oxygen concentration. It is important to distinguish between three different principles in detecting the red luminescence: Intensity (how strong the return is), life-time (how quickly the return dies out) and phase shift (in principle also a life time based measurement, see below Measurement Principle). Intensity based measurements are technically easier to do, but they can drift over time. The different signal detection techniques are summarized by Wolfbeis (1991), Demas et al. (1999) and Glud et al. (2000) along with a wide range of applications. The function and use of lifetime-based optodes was described by Holst et al. (1995) and Klimant et al. (1996).
Measurement principle
The “Oxygen Optode” from Aanderaa Instruments is based on oxygen luminescence quenching of a platinum porphyries complex. The lifetime and hence the oxygen measurement is made by a so-called phase shift detection of the returning, oxygen quenched red luminescence. The relationship between oxygen concentration and the luminescent decay time can be described by the Stern- Volmer equation:
Where: τ = decay time, τ0 = decay time in the absence of oxygen and KSV = Stern-Volmer constant (the quenching efficiency). The foil is excited with a blue-green light modulated at 5 kHz. The decay time is a direct function of the phase of the received red light, which is used, directly for oxygen detection, without calculating the decay time. The basic working principles of dynamic fluorescence quenching, lifetime-based optodes and phase shift detection can be found in e.g. Klimant et al. (1996); Demas et al. (1999); Glud et al. (2000). The sensor housing is made of Titanium, rated to 6000 dbar pressure, with a diameter of 36 mm and a total length of 86 mm. This housing includes an optical part (Fig. 1 a temperature sensor and the necessary electronics (a microprocessor with digital signal processing capacity) to process signals and output absolute temperature compensated oxygen readings (in M or % saturation). The sensing foil is composed of the oxygen sensitive fluorescent substance (luminophore) that is embedded in a polymer layer, which is coated onto a thin film of polyester support. The most commonly used oxygen luminophores have been ruthenium complexes (e.g. Klimant et al.) but for this sensor an oxygen sensitive luminophore based on a platinum porphyrine complex, commercial available from PreSens GmbH (Regensburg, Germany) was used due to its higher dynamics. Two types of foils, with and without, a gas permeable protective black silicon layer is available (Fig. 2). The silicon layer also acted as an optical isolation layer to avoid potential influence from fluorescent material in the surrounding water and/or direct incoming sunlight, when measuring in the photic zone. The disadvantage of this layer is that the sensor response time becomes longer.
References
See also
Internal links
- Instruments and sensors to measure environmental parameters
- Ships of opportunity and ferries as instrument carriers
External links
References
Please note that others may also have edited the contents of this article.
|