Difference between revisions of "Fluorescence sensors"
Cliviahaese (talk | contribs) |
Dronkers J (talk | contribs) |
||
| (17 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Fluorescence sensors can be used to study chlorophyll and to measure dissolved oxygen concentrations. In this article an explanation of the processes in a fluorescence sensor is given as well as an explenation of the sensor design. Next several application op the sensor are mentioned. | |
==Introduction== | ==Introduction== | ||
| − | ''Fluorescence'' is a process in which a photon is absorbed by an atom or molecule and re-emitted at a lower energy (and longer wavelength). The emission of the lower-energy photon generally occurs on a time scale of nano- or pico-seconds, and the change in wavelength is called the [http://de.wikipedia.org/wiki/Stokes-Shift Stokes shift] (Lakowicz 2006)<ref>Lakowicz, J. R. 2006. Principles of Fluorescence Spectroscopy. Springer, 954 pp.</ref>. The ''quantum yield'' of fluorescence is defined as the ratio of the numbers of long-wavelength photons emitted to short-wavelength photons absorbed, and this yield can be strongly modified (quenched) by the chemical environment in which the process occurs. Most ''submersible fluorometers'', including the Fast Repetition Rate and Pulse Amplitude Modulated instruments used to study [[photosynthesis]], emit flashes of excitation light that are micro- or milli- seconds in duration (Roettgers 2007)<ref>Roettgers, R. 2007. Comparison of different variable chlorophyll a fluorescence techniques to determine photosynthetic parameters of natural phytoplankton. Deep-Sea Research, Part I-Oceanographic Research Papers 54: 437-451.</ref>. These flashes are considerably longer than the lifetime of an individual fluorescence decay event, and therefore measure ‘steady state fluorescence’ in the terminology of the photochemist. Fluorescence lifetimes can be measured ''in situ'' using phase-shift techniques, and these have been successfully used to study chlorophyll a ''in vivo'' (Ciencia) and to measure dissolved oxygen concentrations through fluorescence quenching in optodes ([http://www.aanderaa.com/ Aanderaa]). | + | ''Fluorescence'' is a process in which a photon is absorbed by an atom or molecule and re-emitted at a lower energy (and longer wavelength). The emission of the lower-energy photon generally occurs on a time scale of nano- or pico-seconds, and the change in wavelength is called the [http://de.wikipedia.org/wiki/Stokes-Shift Stokes shift](Lakowicz 2006)<ref>Lakowicz, J. R. 2006. Principles of Fluorescence Spectroscopy. Springer, 954 pp.</ref>. The ''quantum yield'' of fluorescence is defined as the ratio of the numbers of long-wavelength photons emitted to short-wavelength photons absorbed, and this yield can be strongly modified (quenched) by the chemical environment in which the process occurs. Most ''submersible fluorometers'', including the Fast Repetition Rate and Pulse Amplitude Modulated instruments used to study [[photosynthesis]], emit flashes of excitation light that are micro- or milli- seconds in duration (Roettgers 2007)<ref>Roettgers, R. 2007. Comparison of different variable chlorophyll a fluorescence techniques to determine photosynthetic parameters of natural phytoplankton. Deep-Sea Research, Part I-Oceanographic Research Papers 54: 437-451.</ref>. These flashes are considerably longer than the lifetime of an individual fluorescence decay event, and therefore measure ‘steady state fluorescence’ in the terminology of the photochemist. Fluorescence lifetimes can be measured ''[[in situ]]'' using phase-shift techniques, and these have been successfully used to study chlorophyll a ''in vivo'' ([http://www.ciencia.com/ Ciencia] in the US) and to measure dissolved oxygen concentrations through fluorescence quenching in optodes ([http://www.aanderaa.com/ Aanderaa]). |
==Sensor design== | ==Sensor design== | ||
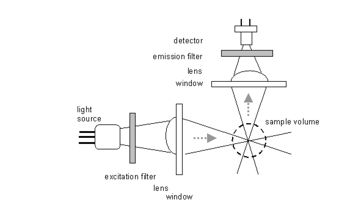
| − | [[image:flu.jpg|thumb| | + | [[image:flu.jpg|thumb|350px|right|Fig. 1: Schematic diagram of a traditional submersible fluorometer.]] |
Until recently, a ''submersible fluorometer'' consisted of 5 main optical components: a light source, a lens system to convey the exciting light to the sample volume, a second lens system to gather the emitted fluorescence, one or more optical filters to separate the excitation and emission wavelengths, and a photodetector. This arrangement is shown schematically in Figure 1. The first submersible instruments used xenon flash lamps and photomultiplier detectors, but these have been replaced by light-emitting diodes and silicon detectors for most applications with considerable savings in instrument size and power consumption. The main optical problem encountered is the tendency of ambient light, or excitation scattered back from the sample volume, to interfere with the measurement of the relatively weak fluorescence signal. Modern compact fluorometers use a wide range of proprietary optical configurations, including coaxial arrangements of the excitation and collection optics or light pipes rather than lenses. | Until recently, a ''submersible fluorometer'' consisted of 5 main optical components: a light source, a lens system to convey the exciting light to the sample volume, a second lens system to gather the emitted fluorescence, one or more optical filters to separate the excitation and emission wavelengths, and a photodetector. This arrangement is shown schematically in Figure 1. The first submersible instruments used xenon flash lamps and photomultiplier detectors, but these have been replaced by light-emitting diodes and silicon detectors for most applications with considerable savings in instrument size and power consumption. The main optical problem encountered is the tendency of ambient light, or excitation scattered back from the sample volume, to interfere with the measurement of the relatively weak fluorescence signal. Modern compact fluorometers use a wide range of proprietary optical configurations, including coaxial arrangements of the excitation and collection optics or light pipes rather than lenses. | ||
==Applications== | ==Applications== | ||
| − | Measurements of fluorescence have been used for many years in marine science to measure concentrations of chlorophyll a and related phaeopigments, but the calibration of these measurements is rendered problematic by natural variations in the quantum yield in living phytoplankton cells (Strass 1990)<ref>Strass, V. 1990. On the calibration of large-scale fluorometric chlorophyll measurements from towed undulating vehicles. Deep-Sea Research, Part A-Oceanographic Research Papers 37: 525-540.</ref>. Fluorometers are also widely used in tracer experiments to study processes of diffusion and mixing, since fluorescent dyes such as rhodamine and fluoresceine are detectable at very low concentrations in natural waters. The recent availability of efficient solid state sources in a wide range of wavebands, including the ultra-violet (UV), has led to the introduction of fluorescence sensors for other photopigments (phycoerythrin and phycocyanin) as well as coloured dissolved organic matter (CDOM) and hydrocarbons. It has also opened the way for new developments such as the use of multiple excitation wavelengths for discriminating between phytoplankton taxa (Paresys et al 2005)<ref>Paresys, G., Rigart, C., Rousseau, B., Wong, A. W. M., Fan, F., Barbier, J. P., Lavaud, J. 2005. Quantitative and qualitative evaluation of phytoplankton communities by trichromatic chlorophyll fluorescence excitation with special focus on cyanobacteria. Water Research 39: 911-921.</ref>. Excitation and emission wavebands for substances commonly measured using their fluorescence properties are listed in Table 1. | + | Measurements of fluorescence have been used for many years in marine science to measure concentrations of chlorophyll a and related phaeopigments, but the calibration of these measurements is rendered problematic by natural variations in the quantum yield in living [[phytoplankton]] cells (Strass 1990)<ref>Strass, V. 1990. On the calibration of large-scale fluorometric chlorophyll measurements from towed undulating vehicles. Deep-Sea Research, Part A-Oceanographic Research Papers 37: 525-540.</ref>. Fluorometers are also widely used in tracer experiments to study processes of diffusion and mixing, since fluorescent dyes such as rhodamine and fluoresceine are detectable at very low concentrations in natural waters. The recent availability of efficient solid state sources in a wide range of wavebands, including the ultra-violet (UV), has led to the introduction of fluorescence sensors for other photopigments (phycoerythrin and phycocyanin) as well as coloured dissolved organic matter (CDOM) and hydrocarbons. It has also opened the way for new developments such as the use of multiple excitation wavelengths for discriminating between [[phytoplankton]] taxa (Paresys et al 2005)<ref>Paresys, G., Rigart, C., Rousseau, B., Wong, A. W. M., Fan, F., Barbier, J. P., Lavaud, J. 2005. Quantitative and qualitative evaluation of phytoplankton communities by trichromatic chlorophyll fluorescence excitation with special focus on cyanobacteria. Water Research 39: 911-921.</ref>. Excitation and emission wavebands for substances commonly measured using their fluorescence properties are listed in Table 1. |
| − | {| border="1" cellspacing="0" | + | {| border="1" cellspacing="0" width="400px" cellpadding=4 style="margin: 0 auto" |
| − | |+ Table 1: Approximate excitation and emission wavebands of the main substances | + | |+ Table 1: Approximate excitation and emission wavebands of the main substances measured by submersible fluorometers in the marine environment. |
|- | |- | ||
| − | ! Substance | + | ! align="left" |Substance |
! Excitation (nm) | ! Excitation (nm) | ||
! Emission (nm) | ! Emission (nm) | ||
|- | |- | ||
| Chlorophyll a | | Chlorophyll a | ||
| − | | 400-450 | + | | align="center" |400-450 |
| − | | 650-750 | + | | align="center" |650-750 |
|- | |- | ||
| Phycoerythrins | | Phycoerythrins | ||
| − | | 545-565 | + | | align="center" |545-565 |
| − | | 565-585 | + | | align="center" |565-585 |
|- | |- | ||
| Phycocyanins | | Phycocyanins | ||
| − | | 615-650 | + | | align="center" |615-650 |
| − | | 640-660 | + | | align="center" |640-660 |
|- | |- | ||
| CDOM (typical) | | CDOM (typical) | ||
| − | | 360-390 | + | | align="center" |360-390 |
| − | | 450-470 | + | | align="center" |450-470 |
|- | |- | ||
| Hydrocarbons | | Hydrocarbons | ||
| − | | 250-350 | + | | align="center" |250-350 |
| − | | 300-450 | + | | align="center" |300-450 |
|- | |- | ||
| Fluorescein | | Fluorescein | ||
| − | | 410-510 | + | | align="center" |410-510 |
| − | | 500-600 | + | | align="center" |500-600 |
|- | |- | ||
| Rhodamine B | | Rhodamine B | ||
| − | | 490-590 | + | | align="center" |490-590 |
| − | | 510-690 | + | | align="center" |510-690 |
|} | |} | ||
| − | |||
==See also== | ==See also== | ||
| Line 51: | Line 50: | ||
===Internal Links=== | ===Internal Links=== | ||
* [[Instruments and sensors to measure environmental parameters]] | * [[Instruments and sensors to measure environmental parameters]] | ||
| + | * [[Differentiation of major algal groups by optical absorption signatures]] | ||
* [[Optical measurements in coastal waters]] | * [[Optical measurements in coastal waters]] | ||
| − | |||
* [[Light fields and optics in coastal waters]] | * [[Light fields and optics in coastal waters]] | ||
| + | |||
| + | ===External Links=== | ||
| + | *[http://en.wikipedia.org/wiki/Fluorescence Fluorescence on Wikipedia] | ||
==References== | ==References== | ||
| Line 64: | Line 66: | ||
|AuthorName=Alex.cunningham}} | |AuthorName=Alex.cunningham}} | ||
| − | [[Category: | + | [[Category:Coastal and marine observation and monitoring]] |
| − | + | [[Category:Observation of biological parameters]] | |
| − | [[Category: | ||
Latest revision as of 16:52, 29 June 2019
Fluorescence sensors can be used to study chlorophyll and to measure dissolved oxygen concentrations. In this article an explanation of the processes in a fluorescence sensor is given as well as an explenation of the sensor design. Next several application op the sensor are mentioned.
Contents
Introduction
Fluorescence is a process in which a photon is absorbed by an atom or molecule and re-emitted at a lower energy (and longer wavelength). The emission of the lower-energy photon generally occurs on a time scale of nano- or pico-seconds, and the change in wavelength is called the Stokes shift(Lakowicz 2006)[1]. The quantum yield of fluorescence is defined as the ratio of the numbers of long-wavelength photons emitted to short-wavelength photons absorbed, and this yield can be strongly modified (quenched) by the chemical environment in which the process occurs. Most submersible fluorometers, including the Fast Repetition Rate and Pulse Amplitude Modulated instruments used to study photosynthesis, emit flashes of excitation light that are micro- or milli- seconds in duration (Roettgers 2007)[2]. These flashes are considerably longer than the lifetime of an individual fluorescence decay event, and therefore measure ‘steady state fluorescence’ in the terminology of the photochemist. Fluorescence lifetimes can be measured in situ using phase-shift techniques, and these have been successfully used to study chlorophyll a in vivo (Ciencia in the US) and to measure dissolved oxygen concentrations through fluorescence quenching in optodes (Aanderaa).
Sensor design
Until recently, a submersible fluorometer consisted of 5 main optical components: a light source, a lens system to convey the exciting light to the sample volume, a second lens system to gather the emitted fluorescence, one or more optical filters to separate the excitation and emission wavelengths, and a photodetector. This arrangement is shown schematically in Figure 1. The first submersible instruments used xenon flash lamps and photomultiplier detectors, but these have been replaced by light-emitting diodes and silicon detectors for most applications with considerable savings in instrument size and power consumption. The main optical problem encountered is the tendency of ambient light, or excitation scattered back from the sample volume, to interfere with the measurement of the relatively weak fluorescence signal. Modern compact fluorometers use a wide range of proprietary optical configurations, including coaxial arrangements of the excitation and collection optics or light pipes rather than lenses.
Applications
Measurements of fluorescence have been used for many years in marine science to measure concentrations of chlorophyll a and related phaeopigments, but the calibration of these measurements is rendered problematic by natural variations in the quantum yield in living phytoplankton cells (Strass 1990)[3]. Fluorometers are also widely used in tracer experiments to study processes of diffusion and mixing, since fluorescent dyes such as rhodamine and fluoresceine are detectable at very low concentrations in natural waters. The recent availability of efficient solid state sources in a wide range of wavebands, including the ultra-violet (UV), has led to the introduction of fluorescence sensors for other photopigments (phycoerythrin and phycocyanin) as well as coloured dissolved organic matter (CDOM) and hydrocarbons. It has also opened the way for new developments such as the use of multiple excitation wavelengths for discriminating between phytoplankton taxa (Paresys et al 2005)[4]. Excitation and emission wavebands for substances commonly measured using their fluorescence properties are listed in Table 1.
| Substance | Excitation (nm) | Emission (nm) |
|---|---|---|
| Chlorophyll a | 400-450 | 650-750 |
| Phycoerythrins | 545-565 | 565-585 |
| Phycocyanins | 615-650 | 640-660 |
| CDOM (typical) | 360-390 | 450-470 |
| Hydrocarbons | 250-350 | 300-450 |
| Fluorescein | 410-510 | 500-600 |
| Rhodamine B | 490-590 | 510-690 |
See also
Internal Links
- Instruments and sensors to measure environmental parameters
- Differentiation of major algal groups by optical absorption signatures
- Optical measurements in coastal waters
- Light fields and optics in coastal waters
External Links
References
- ↑ Lakowicz, J. R. 2006. Principles of Fluorescence Spectroscopy. Springer, 954 pp.
- ↑ Roettgers, R. 2007. Comparison of different variable chlorophyll a fluorescence techniques to determine photosynthetic parameters of natural phytoplankton. Deep-Sea Research, Part I-Oceanographic Research Papers 54: 437-451.
- ↑ Strass, V. 1990. On the calibration of large-scale fluorometric chlorophyll measurements from towed undulating vehicles. Deep-Sea Research, Part A-Oceanographic Research Papers 37: 525-540.
- ↑ Paresys, G., Rigart, C., Rousseau, B., Wong, A. W. M., Fan, F., Barbier, J. P., Lavaud, J. 2005. Quantitative and qualitative evaluation of phytoplankton communities by trichromatic chlorophyll fluorescence excitation with special focus on cyanobacteria. Water Research 39: 911-921.
Please note that others may also have edited the contents of this article.
|