Harmful algal bloom
Definition of Harmful Algal Bloom (HAB):
Harmful algal blooms or HABs are algal blooms composed of phytoplankton that naturally produce biotoxins. Harmful algal blooms (HABs) can occur in marine, estuarine, and fresh waters.
The term 'harmful algal bloom' is sometimes used to designate any phytoplankton bloom event that causes 'negative' impacts on the marine ecosystem, for example oxygen depletion or sunlight shading. This is the common definition for Harmful Algal Bloom (HAB), other definitions can be discussed in the article
|
This article deals with toxic algal blooms: effects, environmental conditions, factors that promote HABs and management measures.
Contents
Effects of harmful algal blooms
The toxins produced by harmful algal blooms (HABs) have direct negative impacts on human health and on many marine organisms. Marine HABs further impact on other aspects of human wellbeing, including human commercial and recreational uses of the coastal and marine environments, such as fishing, aquaculture and tourism, and non-market, passive uses of the ocean, such as preferences for particular ecological states.
Most algal toxins are neurotoxins, which can affect the nervous, digestive, respiratory, hepatic, dermatological or cardiac systems. Consumption of toxins bio-accumulated in shellfish produces shellfish poisoning (PS) syndromes such as [1] Amnesic SP, Azaspirazid SP, Diarrhetic SP, Neurotoxic SP and Paralytic SP. Toxins in fish can produce Ciguatera Fish Poisoning.
Beach visitors can experience serious health problems when respiring aerosols containing algal biotoxins[2][3]. Toxic HABs have recently emerged as a potential risk for the contamination of drinking water supplied by desalination systems[4][5][6].
Socio-economic costs cannot easily be quantified, but they are considerable[7][1][8].
Estimates are in the order of several billon US$ annually up to about 8 billion US$, related to precautionary closure of mariculture farms, reduced attractiveness of beaches for coastal tourists and economic impacts of marine phycotoxins on human health.
The greatest direct effect of HABs concerns mariculture. Mariculture has experienced tremendous growth in recent decades and has become a food source on which much of the world's population depends. As the growth of mariculture is expected to continue, harmful algal blooms are an increasing threat. Most shellfish species can eliminate phycotoxins within a few weeks, but retention of some toxins (e.g. saxitoxins) in some species, such as sea scallops (Placopecten magellanicus) and Atlantic surfclams (Spisula solidissima), can last up to 5 years[9]. The paradox is that the waste from finfish farms itself promotes conditions for the development of HABs[10][11].
Conditions favouring the development of harmful algal blooms
HABs are natural phenomena, but these events can be favoured by anthropogenic pressures in coastal areas. It is not known exactly how toxin producing algae develop. What is known, however, is that most toxic algae belong to the class of flagellates and cyanobacteria. Dinoflagellates account for the majority (about 75% of HAB species[12]. Environmental conditions favorable for the development of flagellates and cyanobacteria therefore create the greatest risk for the development of HABs. Among the algae of the diatom class (large plankton with silica cell wall) there are also toxic species, but these are rarer than among the flagellates. The following is known about the shift from conditions favorable for the development of diatoms to conditions favorable for the development of flagellates and cyanobacteria, thus promoting the occurrence of harmful algal blooms:
- Higher temperatures. The optimal growth of diatoms occurs at relatively low temperatures compared to flagellates and cyanobacteria[13]. This is consistent with experiments that show increasing occurrence of HABs with temperature[14]. Warmer waters are thought to also favour smaller-sized cells (less diatoms and more potentially harmful flagellates and cyanobacteria) as they are more efficient in harvesting light and nutrients and maintaining their position in the euphotic zoneCite error: Closing
</ref>missing for<ref>tag. - A high ratio of dissolved nitrogen N versus phosphorus P. This has several causes: (a) Very small cells, such as picocyanobacteria, have a lower requirement for P due to the smaller need for structural components in the cell[15]; (b) Many harmful dinoflagellates are mixotrophic [16][17], which means that they can ingest dissolved and particulate organic material and thus correct an imbalance in the stoichiometric N:P ratio[18][19][20]; (c) Harmful algae can release excess N via toxins [21]. Many cyanobacteria and marine dinoflagellate HABs are more toxic when N is in stoichiometric excess over P. In the dinoflagellate Alexandrium tamarense, saxitoxin production has been shown to increase by three- to fourfold under P deficiency[22]. Increasing N:P ratios in ecosystems therefore shift communities toward systems with trait dominance of higher optimal N:P ratios (higher P uptake affinity and decreasing N retention) which is a typical HAB trait[23].
- Increasing proportions of N in the form of ammonium and urea (CO(NH2)2). Causes: (a) Potentially harmful flagellates and cyanobacteria grow better on ammonium (NH4+) whereas diatoms prefer nitrate (NO3-)[24]; (b) Mixotrophic harmful dinoflagellates can use urea as food source[25].
- Enhancement of stratified conditions[26]. Causes for favouring potentially harmful flagellates and cyanobacteria over diatoms are: (a) Larger phytoplankton sinks more easily out of the photic zone, thus smaller plankton dominates [27]; (b) Many harmful dinoflagellates are mixotrophs which can swim to the pycnocline to capture organic prey[28].
- Reduction of the Si:N ratio. Diatoms require Si for growth; Si limitation favours non-Si species such as flagellates and cyanobacteria[29].
In some cases, however, when there is a risk of toxic blooms of diazotrophic (N2-fixing) cyanobacteria, release of N-rich effluents may be recommended, as diazotrophs thrive when N:P ratios are low.[30]
Causes for an increase in HABs
The majority of HABs (dinoflagellates as well as diatoms) rely on vegetative cells to survive inhospitable conditions. Under suboptimal growth conditions, some highly toxic HABs such as Alexandrium spp. can reproduce sexually and form resting cysts. These cysts settle on sediments and then undergo resuspension during storms or coastal upwelling, enabling (re)colonisation of existing and new areas. Advection and dispersion of HABs, increasing turbulent shear forces breaking up cells, and/or nutrient limitation are all understood to contribute to the termination of HABs[8]. Although no quantitative estimates can be given, there is strong evidence that the occurrence of harmful algal blooms has increased during the past decades. The causes for an increase in HABs are related to the furtherance of the above mentioned conditions favorable to their development. Probable causes are[31]:
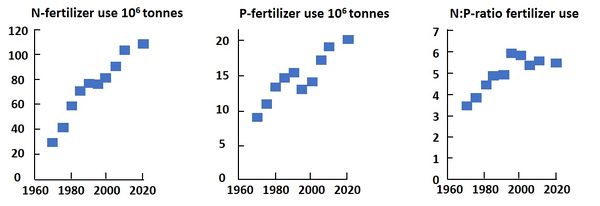
- Increase of nitrogen-rich effluents and atmospheric emissions to the sea. It has been estimated that the atmospheric deposition of nutrients in the ocean is now about 20-fold greater than the Redfield ratio for N:P. The main cause is the increasing use of fertilizers; in the period 1970-2000 the N-fertilizer use has increased much faster than the P-fertilizer use (see Fig. 1) [32]. Only about half of the fertilizer N is taken up by crops; the remainder is partly stored in the soil and partly emitted to the sea via runoff and the atmosphere. Other N-rich sources are the widespread and expanding fish farms, which release N mainly in chemically reduced form (e.g., ammonium, dissolved organic N, DON)[33]. The increase of the N:P ratio is further due to the more efficient reduction of P compared to N in sewage treatment plants and the reduction of P in laundry products.
- Fertilizer effluents and emissions produce a shift from nitrate to ammonium and urea, which favours HABs[21][25].
- Effect of river dams. Large fractions of the fluvial P load (about 43% of total dissolved P and reactive particulate P) and Si load (about 20% of dissolved Si and reactive particulate Si) are bound to sediments that are retained in upstream reservoirs, whereas about 90% of the total N load is unaffected[29][34]. This results in an increase in the N:P and N:Si ratios of riverine delivery to coastal areas following dam construction.
- Effects of climate change: (a) Rising seawater temperatures; (b) Intensification of sea water stratification; (c) Increase in peak river discharges and corresponding increase in nitrogen supply in coastal waters[35]; (d) Increase in nutrient concentrations associated with intensification of upwelling events[36].
- Spreading of harmful algae species across the oceans by increased transport of algae with ship ballast water[1].
Measures for reducing the risk of HABs
The factors that promote the occurrence of HABs are expected to become more important in the future. This holds in the first place for global warming and for eutrophication, in particular the nitrogen component of eutrophication. Efforts to combat harmful algal blooms are vital, but simple solutions do not exist. It is widely recognized that action is needed to halt global climate change and to reduce nitrogen emissions from agriculture. To this end, agreements have been made and initiatives have been developed at various administrative levels. Important international frameworks have been set up for climate policy that will eventually reverse the trend of global warming. A comparable encompassing agreement has not yet been established for agricultural emissions, although in Europe the Nitrates Directive has been in force since 1991. This directive has contributed to a reduction in N emissions from European agriculture[39] - however, without special focus on the nitrate: ammonium ratio of the emissions.
Local reduction of nutrient concentrations can be achieved by harvesting marine products that grow on nutrients and provide economic value (benefit from ecosystem goods and services, see Mariculture) [40][41]. Examples are the harvesting of farmed mussels[42] and the harvesting of seaweed[43]. The restoration of critical coastal habitats (seagrass meadows, coral reefs, oyster reefs, mangrove forests and salt-marshes) also contributes to remove nutrients, increases sequestration of organic matter in benthic sediment, and increases rates of denitrification[44].
Certain measures may contribute to mitigate the impact of HABs (for a more detailed and complete overview see e.g. Berdalet et al., 2016[1] and Wells et al., 2020[45]):
- Development and implementation of new efficient techniques for monitoring HABs and biotoxins and for monitoring marine conditions that are favorable for the development of HABs, in order to improve early warning;
- Management measures for aquaculture to reduce HAB development, for example by timing the harvest, by enhanced flushing and aeration or by relocation to offshore areas where excess N concentrations are less likely to build up;
- Furthering understanding of fundamental aspects of HAB species in terms of toxin production, life cycles and interactions with bacteria in order to develop better targeted measures.
Measures to reduce nutrient inputs to coastal waters not only lead to fewer harmful algal blooms but also to a lower biomass of non-toxic algae. With such measures, aquaculture faces a trade-off between fewer toxic algal blooms and a decline in fisheries productivity.[46]
Measures to eliminate harmful algae, for example through the use of viruses, parasites, grazers or algicides, encounter serious problems due to hazardous side effects[47]. That is why many countries have bans on such measures. Experiments in Korea to remove toxic algae through flocculation using clay particles have reported successful application without harmful side effects[48]. High clay concentrations are needed.
Another, more holistic approach to toxic HAB mitigation experimented in Puget Sound (USA), is the restoration of coastal habitats with seagrass that harbors algicidal bacteria[49][50].
Useful links
Related articles
- Possible consequences of eutrophication
- ALGADEC - Detection of toxic algae with a semi-automated nucleic acid biosensor
- Plankton remote sensing
- Mariculture
- Marine Plankton
- Algal bloom dynamics
- Eutrophication in coastal environments
- Real-time algae monitoring
- The Ocean as an economic area - a competitive Europe
References
- ↑ 1.0 1.1 1.2 1.3 Berdalet, E., Fleming, L.E., Gowen, R., Davidson, K., Hess, P., Backer, L.C., Moore, S.K., Hoagland, P. and Enevoldsen, H. 2016. Marine harmful algal blooms, human health and wellbeing: challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. U.K. 96: 61–91
- ↑ Fleming L.E., Kirkpatrick B., Backer L.C., Walsh C.J., Nierenberg K., Clark J., Reich A., Hollenbeck J., Benson J., Cheng Y.S., Naar J., Pierce R., Bourdelais A.J., Abraham W.M., Kirkpatrick G., Zaias J., Wanner A., Mendes E., Shalat S., Hoagland P., Stephan W., Bean J., Watkins S., Clarke T., Byrne M. and Baden D.G. 2011. Review of Florida red tide and human health effects. Harmful Algae 20: 224–233
- ↑ Berdalet E., Vila M. and Abos-Herrandiz, R. 2015. Expansion of the benthic dinoflagellate Ostreopsis with climate change: health risks assessment and policy strategies for management. Harmful Algal Blooms and Climate Change Scientific Symposium. Goteborg, Sweden, 19–22 May 2015
- ↑ Seubert, E.L., Trussell, S., Eagleton, J., Schnetzer, A., Cetinic, I., Lauri, P., Jones, B.H. and Caron, D.A. 2012. Algal toxins and reverse osmosis desalination operations: laboratory bench testing and field monitoring of domoic acid, saxitoxin, brevetoxin and okadaic acid. Water Research 46: 6563–6573
- ↑ Berman, T. 2013. Transparent exopolymer particles as critical agents in aquatic biofilm formation: implications for desalination and water treatment, Desalination and Water Treatment 51: 4-6
- ↑ Flemming, H.C. and Wingender, J. 2001. Relevance of microbial extracellular polymeric substances (EPSs)--Part I: Structural and ecological aspects. Water Sci Technol. 43(6): 1-8
- ↑ Hoagland, P. and Scatasta, S. 2006. The economic effects of harmful algal blooms. In Graneli E. and Turner J.T. (eds) Ecology of harmful algae. New York, NY: Springer-Verlag, pp. 391–402
- ↑ 8.0 8.1 Ross Brown, A., Lilley, M., Shutler, J., Lowe, C., Artioli, Y, Torres, R., Berdalet, E. and Tyler, C.R. 2020. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 12: 1663-1688
- ↑ Landsberg, J.H. 2002. The effects of Harmful Algal Blooms on aquatic organisms. Reviews in Fisheries Science 10(2): 113–390
- ↑ Anderson, D. 2012. HABs in a changing world: a perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. Harmful Algae 2012 (2012). 2014 ; 2012: 3–17 PMID: 26640829; PMCID: PMC4667985
- ↑ Strain, P. M. and Hargrave, B. T. 2005. Salmon aquaculture, nutrient fluxes and ecosystem processes in Southwestern New Brunswick, in Environmental Effects of Marine Finfish Aquaculture, Handbook of Environmental Chemistry, ed. B. T. Hargrave (Berlin: Springer): 29–57
- ↑ Smayda, T.J. 1997. Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 42: 1137-1153
- ↑ Anderson, N.J. 2000. Diatoms, temperature and climate change. Eur. J. Phycol. 35: 307–314
- ↑ Paerl, H.W. and Scott, J.T. 2010. Throwing fuel on the fire: synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 44: 7756–7758
- ↑ Finkel, Z.V., J. Beardall, K.J. Flynn, A. Quiqq, T.A.V. Rees, and J.A. Raven. 2010. Phytoplankton in a changing world: Cell size and elemental stoichiometry. Journal of Plankton Research 32:119–137
- ↑ Mitra, A. and Flynn, K.J. 2010. Modelling mixotrophy in harmful algal blooms: more or less the sum of the parts? J. Mar. Syst. 83: 58–169
- ↑ Stoecker, D., Tillmann, U. and Graneli, E. 2006. Phagotrophy in harmful algae. In: Graneli, E. and Turner, J. (eds) Ecology of Harmful Algae, Series: Ecological Studies, Vol. 189, Springer Verlag, Heidelberg, pp 177–187
- ↑ Burkholder, J.M., Glibert, P.M. and Skelton, H. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8: 77–93
- ↑ Jeong, H.J., Yoo, Y.D., Kim, J.S., Seong, K.A., Kang, N.S. and Kim, T.H. 2010. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 45: 65–91
- ↑ Flynn, K.J., Stoecker, D.K., Mitra, A., Raven, J.A., Glibert, P.M., Hansen, P.J., Graneli, E. and Burkholder, J.M. 2013. Misuse of the phytoplankton-zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J. Plankt. Res 35: 3–11
- ↑ 21.0 21.1 Glibert, P.M., Wilkerson, F.P., Dugdale, R.C., Raven, J.A., Dupont, C., Leavitt, P.R., Parker, A.E., Burkholder, J.M. and Kana, T.M. 2016. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 61: 165–197
- ↑ Graneli, E., and Flynn, K.J. 2006. Chemical and physical factors influencing toxin content. Pp. 229–241 in Ecology of Harmful Algae. E. Graneli, and J.T. Turner, eds, Springer, Heidelberg, Germany
- ↑ Meunier, C.L., Boersma, M., El-Sabaawi, R., Halvorson, H.M., Herstoff, E.M., Van de Waal, D.B., Vogt, R.J. and Litchman, E. 2017. From elements to function: toward unifying ecological stoichiometry and trait-based ecology. Front. Environ. Sci 5, 18
- ↑ Glibert, P.M. 2017. Eutrophication, harmful algae and biodiversity - challenging paradigms in a world of complex nutrient changes. Mar. Poll. Bull. 124: 591–606
- ↑ 25.0 25.1 Glibert, P.M., Manager, R., Sobota, D.J., Bouwman, L. 2014. The Haber-Bosch-Harmful algal bloom (HB-HAB) link. Environ. Res. Lett. 9, 105001
- ↑ Telesh, I., Schubert, H. and Skarlato, S. 2021. Abiotic stability promotes dinoflagellate blooms in marine coastal ecosystems. Estuarine, Coastal and Shelf Science 251, 107239
- ↑ Winder, M., Reuter, J.E. and Schladow, S.G. 2009. Lake warming favours small-sized plankton diatom species. Proc. Roy. Soc B 276: 427–435
- ↑ Stoecker, D.K., Hansen, P.J., Caron, D.A. and Mitra, A. 2017. Mixotrophy in the marine plankton. Ann. Rev. Mar. Sci. 9: 311–335
- ↑ 29.0 29.1 Maavara, T., Dürr, H.H. and Van Cappellen, P. 2014. Worldwide retention of nutrient silicon by river damming: from sparse data set to global estimate. Glob. Biogeochem. Cycl. 28: 842–855
- ↑ Elmgren, R. and Larsson, U. 2001. Nitrogen and the Baltic Sea: managing nitrogen in relation to phosphorus. Sci. World J. 1: 371-377
- ↑ Glibert, P.M. 2020. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 91, 101583
- ↑ Penuelas, J., Poulter, B., Sardans, J., Ciais, P., van der Velde, M., Bopp, L., et al. 2013. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 4: 2934
- ↑ Bouwman, A.F., Beusen, A.H.W., Glibert, P.M., Overbeck, C., Pawlowski, M., Silveiva, J.H., Mulsow, S., Yu, R. and Zhou, M.J. 2013. Mariculture: significant and expanding cause of coastal nutrient enrichment. Environ. Res. Lett. 8, 044026
- ↑ Maavara, T., Parsons, C.T., Ridenour, C., Stojanovic, S., Dürr, H.H., Powley, H.R. and Van Cappellen, P. 2015. Global phosphorus retention by river damming. Proc. Natl. Acad. Sci. U. S. A. 112, 15603–15608
- ↑ Howarth, R.W., Swaney, D.P., Boyer, E.W., Marino, R., Jaworski, N. and Goodale, C. 2006. The influence of climate on average nitrogen export from large watersheds in the Northeastern United States. Biogeochemistry 79: 163–186
- ↑ Goes, J.I., Thoppil, P.G., Gomes, H.D.R. and Fasullo, J.T. 2005. Warming of the Eurasian landmass is making the Arabian Sea more productive. Science 308: 545–547
- ↑ Glibert, P.M. and Burford, M.A. 2017. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 30: 58–69
- ↑ FAO. 2019. World fertilizer trends and outlook to 2022. FAO, Rome
- ↑ Velthof G.L., Lesschen, J.P., Webb, J., Pietrzak, S., Miatkowski, Z., Pinto, M., Kros, J. and Oenema, O. 2014.The impact of the Nitrates Directive on nitrogen emissions from agriculture in the EU-27during 2000–2008. Science of the Total Environment 468–469: 1225–1233
- ↑ Burkholder, J. M., and Shumway, S.E. 2011. Bivalve shellfish aquaculture and eutrophication, in Shellfish Aquaculture and the Environment, ed. S.E. Shumway (Hoboken, NY: John Wiley & Sons, Inc.), 155–215
- ↑ Petersen, J.K., Holmer, M., Termansen, M. and Hasler, B. 2019. Nutrient extraction through bivalves. In: Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, O. (Eds.), Goods and Services of Marine Bivalves. Springer, Cham, pp. 179–208
- ↑ Kotta, J., Futter, M., Kaasik, A., Liversage, K., Rätsep, M., Barboza, F. R., et al. 2020. Cleaning up seas using blue growth initiatives: mussel farming for eutrophication control in the Baltic Sea. Sci. Total Environ. 709:136144
- ↑ Xiao, X., Agusti, S., Lin, F., Li, K., Pan, Y., Yu, Y., Zheng, Y., Wu, J. and Duarte, C.M. 2017. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Scientific Reports 7: 46613 DOI: 10.1038/srep46613
- ↑ Malone, T.C. and Newton, A. 2020. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 7:670
- ↑ Wells, M.L., Karlson, B., Wulff, A., Kudela, R., Trick, C., Asnaghi, V., Berdalet, E., Cochlan, W.,. Davidson, K., De Rijcke, M., Dutkiewicz, S., Hallegraeff, G., Flynn, K.J., Legrand, C., Paerl, H., Slke, J., Suikkanen, S., Thompson, P. and Trainer, V.L. 2020. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 91, 101632
- ↑ Anderson, D. 2012. HABs in a changing world: a perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. Harmful Algae 2012 (2012). 2014; 2012: 3-17
- ↑ Paerl, H.W. 2018. Mitigating toxic planktonic cyanobacterial blooms in aquatic ecosystems facing increasing anthropogenic and climate pressures. Toxins 10, 76; doi:10.3390/toxins10020076
- ↑ Yu, Z.M., Song, X.X., Cao, X.H. and Liu, Y., 2017. Mitigation of harmful algal blooms using modified clays: theory, mechanisms, and applications. Harmful Algae 69: 48–64
- ↑ Inaba, N., Trainer, V.L., Onishi, Y., Ishii, K., Wyllie-Echeverria, S. and Imai, I. 2017. Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in Puget Sounds, WA, USA. Harmful Algae 62: 136–147
- ↑ Inaba, N., Trainer, V.L., Nagain, S., Kojima, S., Sakami, T., Takagi, S. and Imai, I. 2019. Dynamics of seagrass bed microbial communities used to control artificial Chattonella blooms: a microcosm study. Harmful Algae 84: 139–150
Please note that others may also have edited the contents of this article.
|