In situ monitoring of eutrophication
Introduction
To maximize the usefulness of satellite data and for their calibration and validation it is essential to obtain in situ data for the monitoring of eutrophication. In situ monitoring is the observation and / or measurement of events in its original place (Latin: situs). Oceanographic instruments containing different types of sensors are used to monitor eutrophication in coastal waters. Sensors detect and respond to electrical or optical signals and convert the physical, chemical or biological parameter into a signal which can be measured electrically.
Oceanographic instruments
In this section we focus only on the instruments[1] that measure parameters that are important in the frame of the OSPAR Eutrophication Monitoring Programme: [2]
CTD
The CTD[3][4] - Conductivity, Temperature and Depth - is the standard oceanographic tool for continuously measurement of physical properties of sea water. The instrument contains a cluster of sensors for measuring conductivity, temperature and depth. Depth and salinity are derived from measurements of pressure and electrical conductivity. The CTD is mostly attached to a frame with water-collecting Niskin bottles (CTD rosette). From the deck the rosette is lowered on a cable down to the seafloor and once in the water data are transferred via a conducting cable connecting the CTD to a computer on a ship. The Niskin bottles are closed at predefined depths to target water samples for further analysis. Other sensors to measure chemical or biological parameters such as dissolved oxygen, chlorophyll fluorescence (phytoplankton concentrations) and water light transmission can be added to the cluster.
Expendable bathythermograph
The eXpendable BathyThermograph (XBT) is a free-fall temperature probe providing a profile of measured temperature against depth calculated from a fall-rate model (not by measuring pressure). The XBT is dropped from a ship and measures the temperature (thermistor) as it falls through the water. Two very small copper wires transmit the temperature data to the ship. The depth is calculated from the elapsed time and the expected fall rate. By plotting temperature as a function of depth, scientists obtain a temperature profile of the ocean.
Thermosalinograph
A ThermoSalinoGraph (TSG) is an automated instrument which continuously measures the sea surface temperature and conductivity along the track and on board of the ship using a water intake system (inline measurement). Conductivity and thermistor cells provide the measurements and salinity is derived from these parameters. The TSG is manually turned on once the ship leaves the port. Water flows through the tubes of the instrument which is located in the hull of the vessel. The position of the ship is given by a GPS. A computer collects all data and processes them. Optionally other types of sensors can be added to the instrument for a wider range of measurements.
Continuous Plankton Recorder
The Continuous Plankton Recorder (CPR)[5] is an instrument used to sample and to continuously collect plankton over a long distance at a depth of approximately 10 meters. Water passes through the CPR and plankton are filtered onto a slow-moving band of silk (270 micron mesh size) and covered by a second silk. The silk and plankton are then spooled into a storage tank containing formalin. The silk is divided into samples representing 10 nautical miles of tow. Analysis is done in two ways:
- Determination of the Phytoplankton Colour Index (PCI): the PCI is an in situ measure of ocean colour and a semi quantitative estimate of phytoplankton biomass. The colour of the silk is evaluated against a standard colour chart and given a 'green-ness' value based on the visual discoloration of the CPR silk produced by green chlorophyll pigments.
- Microscopic analysis: individual phytoplankton and zooplankton taxa are identified and counted.
Nutrient analyzers
Oceanographic nutrient measurements are commonly made using traditional methods by collecting water samples with subsequent analysis in the laboratory. Disadvantages of this monitoring are: costly, quality degradation of the sample and limited temporal resolution. Nutrient analyzers are used in situ to measure nutrients such as nitrate, nitrite, ammonia, phosphate, silicate. Most in situ nutrient sensors use wet chemical techniques based on laboratory methods. Alternatives include optical analyzers such as nitrate (an important nutrient in the development of algal blooms) detection using UV-absorbance. The wet chemical nutrient analyzers require the addition of chemical reagents to determine the nutrients. The resulting solution develops a particular property (e.g. color) depending on the concentration of the target nutrient, which then can be measured. The UV technique does not require reagents or waste storage but measures nitrate concentrations based on the absorption characteristics of nitrate in the ultraviolet spectrum (200-400 nm).
Sensors
In this section we focus only on sensors that measure parameters that are important in the frame of the OSPAR Eutrophication Monitoring Programme: [2]
Temperature
The simplest mechanical way to measure temperature is by using a mercury-in-glass thermometer. They are commonly used to measure sea surface temperature by placing it in a bucket of sea water. Electrical temperature sensors such as the Resistance Temperature Detectors (RTDs) and the thermistors are more frequently used on a CTD. A thermistor is a type of resistor composed of a small piece of electrically semiconductor material (metallic oxides) such as a resistor which exhibits a large change in resistance proportional to a small change in temperature (negative temperature coefficient). RTDs are sensors used to measure temperature by correlating the resistance of the RTD element (pure metals usually platinum) with temperature (positive temperature coefficient).
Turbidity
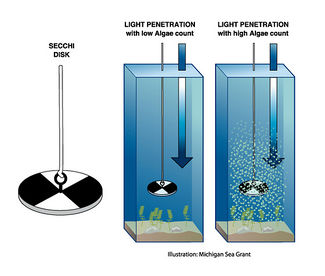
Turbidity is a measure of water clarity or transparency caused by suspended particles from organic (algae, plankton) or inorganic (fine silts or clays) origin. The more turbid the water the less light is transmitted. A common simple and cheap device for manually measuring ocean turbidity is the Secchi disk which consists of a circular disk (20-30 cm in diameter). The disc is being lowered into the water and the depth at which the disc is no longer visible, is a measure of the clarity of the water and is known as the Secchi depth and is related to water turbidity. The Secchi disk readings do not provide an exact measure of transparency (for example: interpretation of different observers) and are often replaced by the use of turbidity sensors. Turbidity sensors designed for extended in situ measurements are based on nephelometric or optical-backscatter principles where the scattering and absorbing effects of the suspended particles on light are measured [6]. Nephelometers measure the concentration of suspended particles in a liquid by employing a light beam 90 degrees from the light detector. Particle density is then a function of the light reflected into the detector from the particles. Optical-backscatter sensors (OBS) measure the same properties as nephelometers but the angle between the light source and the detector is less than 90 degrees.
Salinity
Salinity is a measure of the quantity of salt in a volume of sea water and can be measured in situ using a conductivity sensor on a CTD. The sensor measures the electrical conductivity (electrical current: ions in solution that flow through the sea water (dissolved salts conduct more electricity than water without salts)) at a known temperature and pressure and this conductivity is converted to salinity using a formula.
Dissolved oxygen
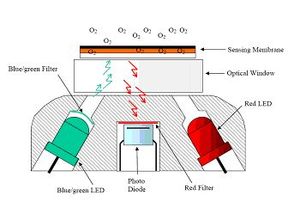
Dissolved oxygen (DO) is the amount of oxygen dissolved in the water. Traditional measurement of oxygen is based on the wet chemistry technique (Winkler titration method). This method is very accurate but dependent on the quality of experience, chemicals and equipment. For in situ measurements two types of sensors are used: electrochemical sensors (Clark-type sensors) and optical sensors (optodes). The principle of electrochemical sensors is that dissolved oxygen diffuses through a membrane and reaches a cathode where the oxygen is reduced. The electric current between cathode and anode is measured and is correlated to oxygen concentration. Optode measurements work according to the principle of dynamic fluorescence quenching. Quenching refers to any process which decreases the florescence intensity of a given substance[8]. The sensors contain fluorescent dye that is excited by light of a certain wavelength. Depending on the amount of oxygen molecules present the luminescence response of the optical sensor varies. A polymer optical fiber transmits the excitation light of the sensor and at the same time also transmits the fluorescence response of the sensor to the measurement device. For example, with oxygen, if a ruthenium-complex is illuminated with a blue LED, it will be excited and emit back a red luminescent light with an intensity, or lifetime, which directly depends on the ambient oxygen concentration.
Phytoplankton chlorophyll a
An important consequence of the enrichment of nutrients (eutrophication) is the growth of algae and other phytoplankton. These algae contain chlorophyll, an important biochemical component that is responsible for photosynthesis. Chlorophyll a is the most abundant form of chlorophyll within photosynthetic organisms and gives plants their green color. The amount of chlorophyll found in a water sample is used to estimate the concentration of phytoplankton (biomass). Phytoplankton emits light in the red portion of the spectrum called fluorescence. Monitoring fluorescence can help scientists describe the physiological state of phytoplankton, determine the cause of population decreases, and make accurate estimates of primary productivity. For in situ monitoring fluorescence sensors or fluorometers are used to induce chlorophyll fluorescence by shining a beam of light of the proper wavelength into the water and then measuring the higher wavelength light which is emitted [9]. In situ measurements are only rough approximations of chlorophyll a concentrations and need to be enhanced through laboratory (extraction) methods.
More information
- Continuous Plankton Recorder (CPR)
- Nutrient analysers
- Temperature sensors
- Salinity sensors
- Turbidity sensors
- Optical backscatter point sensor (OBS)
- Oxygen sensors
- Fluorescence sensors
See also
References
- ↑ Oceanographic Instrumentation, Australian Oceanographic Data Centre, Data Management Group DMG DOC No: 4/98Oceanographic Instrumentation Australia
- ↑ 2.0 2.1 OSPAR Eutrophication Monitoring Programme https://www.ospar.org/work-areas/hasec/eutrophication
- ↑ Woods Hole instruments
- ↑ https://noc.ac.uk/technology/technology-development/instruments-sensors NOC instruments-sensors]
- ↑ CPR sampling: the technical background, materials and methods, consistency and comparability. Batten, S.D.; Clark, R.; Flinkman, J.; Hays, G.; John, E.; John, A.W.G.; Jonas, T.; Lindley, J.A.; Stevens, D.P.; Walne, A. (2003). Progress in Oceanography 58(2-4): 193-215.
- ↑ Rasmussen, P.P., Gray, J.R., Glysson, G.D., and Ziegler, A.C., 2009, Guidelines and procedures for computing time-series suspended-sediment concentrations and loads from in-stream turbidity-sensor and streamflow data: U.S. Geological Survey Techniques and Methods book 3, chap. C4, 53 p.
- ↑ Tengberg A., J. Hovdenes, J. H. Andersson, O. Brocandel, R. Diaz, D. Hebert, T. Arnerich, C. Huber, A. Körtzinger, A. Khripounoff, F. Rey, C. Rönning, S. Sommer and A. Stangelmayer (2006) Evaluation of a life time based optode to measure oxygen in aquatic systems. Limnology and Oceanography, Methods, 4, 7-17.
- ↑ http://en.wikipedia.org/wiki/Quenching_%28fluorescence%29
- ↑ YSI, Technical Notes, The Basics of Chlorophyll Measurement[1]
Please note that others may also have edited the contents of this article.
|