Meiofauna of Sandy Beaches
Contents
The sandy beach biotope
Sandy sediments are a dominant sea shore type along the European coast. They are of prime importance for human recreation, tourism and coastal development. Sandy beaches are more popular than any other type of seashore. They provide the most productive fishing grounds, are major sources for a variety of raw materials (oil, gas and minerals). While the economic and social values of beaches are generally regarded as paramount, sandy shores also have special ecological features and contain a distinctive biodiversity that is often not recognized. Beaches also provide unique ecological services, such as filtration of seawater. The intrinsic ecological values and functions of beaches are often perceived as secondary to their economic value. Due to the lack of quantitative data, the ecological functions of permeable sands is not well represented in coastal management and monitoring programs, and public and policy makers are not aware of its importance. Consequently, these environments and their resources are not protected sufficiently relative to their socio-economical value.
Beach sediments consist of sand particles which are mostly round due to abrasion. Spaces between them occupy typically 30-40% of the sediment volume. They create a system of interstices which is filled with water, air, detritus and organisms. Sand beaches may often appear dead and inhospitable, like a desert (Figure 1). However, a wide variety of organisms inhabit the space between the sediment particles, in damp sand, on sandy shore, among them most abundant – meiofauna communities. A full array of organisms from bacteria, fungi, microphythobenthos, and protozoa to extremely specialized metazoan animals live in the beach sand. Hundreds of species inhabit sand beaches but most of them are small (less than a few mm) and buried. They occupy interstitial spaces between sand grains (Figure 2). The interstitial system is also habitat for larvae and juveniles of certain macrofaunal species.
A key feature of sandy sediments is their permeability which means that water can flow through the interstices. The deflection of bottom water currents (due to tides, wind, or waves) by bottom topography (ripples, animal mounds, stones, etc.) creates horizontal pressure gradients at the sediment surface, which leads to advective flow of water through the sediment [1][2][3].
Meiofauna
Marine meiofauna, defined as animals of microscopic size living in marine sediments from the beach to the deep sea, is one the earth’s richest and most diverse community. Marine meiofauna still contains numerous undescribed species and higher taxa.
The term 'Meiofauna' is related to microscopically small benthic invertebrates that live in both marine and fresh water environments. Meiofauna is formally defined as a group of organisms by their size, larger than microfauna but smaller than macrofauna. In practice these are metazoans (some researchers include protozoans as well) animals that can pass unharmed through a 0.5 – 1 mm mesh but will be retained by a 30 μm mesh, but the exact dimensions will vary from researcher to researcher. Nowadays the term meiofauna is used interchangeably with meiobenthos. Meiofauna is mainly found in and on soft sediments, but also on underwater algae and higher plants as well as on other hard substrates. The heterogeneity of meiofaunal habitats is very large and meiobenthic taxa highly diverse.
Meiofauna Abundance and Distribution
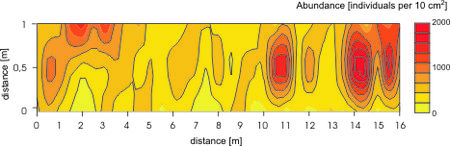
Large numbers of microscopic organisms occupy the system of interstitial canals. The average abundance of metazoan organisms amounts to a few million individuals per square meter. The abundance values vary according to season, latitude, exposure, grain size etc. Populations of sandy beach animals are not randomly distributed. The majority of the meiofauna is found in the upper 2 cm layer. This vertical zonation is mainly controlled by redox potential discontinuity level. There is also horizontal zonation. Across sandy habitats species are often restricted to certain zones. It is well known that meiofauna is patchy distributed, even on very small scale, but the causes of such distributions are still not fully clear (Figure 3).
Adaptations to the sandy beach biotope
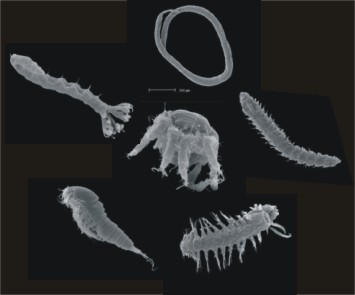
The smallest animals living in the sediment, the interstitial communities, move through the sediment, using the film of water which surrounds individual particles of sand. Interstitial animals therefore display numerous adaptations to resist this very harsh environment. Sandy beaches are dynamic and sensitive places where life is under pressure, practically without a break. Organisms living here must be able to withstand the marine conditions of inundation by salt water which alternates with exposure to terrestrial conditions. These sandy beach dwellers exhibit remarkable physiological and behavioural adaptation to changing environmental conditions. Special morphological adaptations evolved, especially in meiofauna living in the intertidal zone which is under a strong abiotic regime. Certain higher taxa evolved exclusively in the marine interstitial system. Evolutionary constrains caused elaborated life-cycles, migration patterns, special reproductive behaviours and structural adaptations. Sand meiofauna is adapted to:[4]
- narrow spaces, by miniaturization, elongation and flexibility;
- the mobile environment, by adhesion, special locomotion and reinforcing structures;
- the three-dimensional dark conditions, by static organs, reduction of pigments and eyes.
These adaptations undoubtedly are most marked in the interstitial fauna of sand (Figure 4). Interstitial organisms also show other specific adaptation related to reproduction and development – the production of only few eggs, direct sperm transfer or internal fertilization, brood protection, abbreviated larval life and restricted propagation.
Biodiversity of sandy shores
In coastal environments the interactions between coastal morphology, land-ocean exchanges, meteorological and tidal conditions, create a highly complex and finely scaled network of environmental boundaries. These boundary conditions explain why coastal waters have both higher species richness and a richer ecosystem than their oceanic counterparts [5]. Sandy shores are among the ‘simplest’ systems in terms of habitat complexity in comparison to other coastal ecosystems as, for example, rocky shores, algae and seagrass beds. Biodiversity and biomass of interstitial organisms are rather low. However, recent findings have shown that marine sands transfer energy very effectively, and that chemical and biological reactions take place faster there than in fine-grained sediments[6].
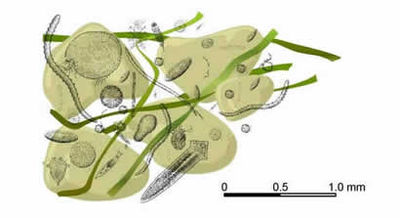
Practically most classes of the Metazoa are represented in the meiofauna of sandy beaches, while the Protozoa are represented by their largest forms, e.g., Foraminifera and Ciliata. The smaller protozoans are generally considered as microfauna. On most beaches, the interstitial fauna is rich and diverse, even exceeding the macrofauna biomass. There may be as many as 25 meiofauna species for every macrofauna species [7]. The reason is greater stability and complexity of the interstitial habitat.
Most common metazoan phyla represented in the benthic meiofauna (Figure 5):
- Cnidaria - Hydroida, Scyphozoa, Anthozoa
- Platyhelminthes – Turbellaria
- Gnathifera – Gnathostomulida, Rotifera, Rotatoria
- Nemertinea
- Nemathelminthes – Nematoda, Kinorhyncha, Priapulida, Loricifera, Gastrotricha
- Tardigrada
- Crustacea – Cephalocarida, Ostracoda, Mystacocarida, Copepoda (e.g. Harpacticoida, Cyclopoida), Malacostraca (e. g. Tanaidacea, Isopoda, Amphipoda)
- Chelicerata – Acari
- Annelida – Polychaeta, Oligochaeta
- Sipuncula
- Mollusca – Aplacophora, Gastropoda
- Tentaculata – Bryozoa
- Echinodermata - -Holothuroidea
- Chaetognatha
- Tunicata – Ascidiacea
This list of meiofauna taxa is far from complete and will getting longer; recent investigations of high-ranking taxa have brought meiofauna into the mainstream of invertebrate phylogeny[4].
Meiofauna is the best-studied component of the interstitial biota. These small creatures are considered as temporary meiofauna[8]. McIntyre[9] used the term permanent members (or permanent meiofauna) for species belonging to the meiofauna during the whole of their life cycle in marine systems in contrast to temporary meiofauna. The dominant taxa of sandy beach meiofauna are nematodes and harpacticoid copepods, with other important group including turbelarians, oligochaetes, ostracods or gastrotrichs.
Ecosystem role of meiofauna
Meiofauna fulfil the following functions:
- they facilitate biomineralization of organic matter and enhance nutrient regeneration;
- they serve as food for a variety of higher trophic levels;
- they exhibit high sensitivity to anthropogenic inputs, making them excellent sentinels of pollution.
Meiofauna, particularly copepods, are preferred foods for a variety of predators, especially juvenile fish, as the meiofaunal copepods are high in the essential fatty acids required by fish. While meiofauna are integral parts of marine food webs, there is no evidence that they are biologically controlled. Top down (predation) control does not regulate meiofaunal assemblages. Meiofauna reproduce so rapidly and are so abundant that predators cannot significantly reduce population size. Food quantity (bottom-up control) also does not appear to limit meiofaunal abundance.
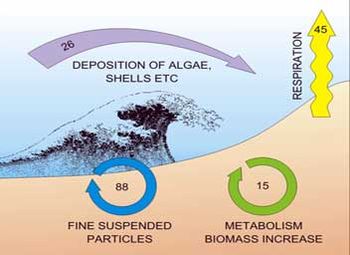
As the light color reveals, organic matter and nutrient pools in near-shore sands are rather low. This may give to scientists and coastal managers the impression that these sediments are biogeochemically inactive. Recent investigations indicate that the contribution of permeable sands in the coastal cycles of matter may be underestimated[10]. Sandy beaches can be as biogeochemically active as organically rich fine-grained sediments. Oxygen and nutrient fluxes can reach similar magnitudes, but high pore water transport rates in these permeable sediments prevent oxygen depletion and limit solute accumulation in the pore water[11]. Meiofauna play an important role as a trophic link between bacteria and larger fauna. They enhance the rate of carbon mineralisation by stimulating microbial activity through predation, and/or consumption of detritus by larger deposit-feeding invertebrates. Bacteria, fungi and other microorganisms living in sand are able to decompose organic matter very efficiently. In this process, decaying algae are reduced to CO2 and biogenic substances. Each square meter of beach burns some 30 kg of organic material per year (Figure 6).
Most contaminants reside in sediments, and meiofauna are intimately associated with sediments over their entire life-cycle, thus they are increasingly being used as pollution sentinels. Because meiofauna have short lifecycles, the effects of a contaminant on the entire life-history can be assessed within a relatively short time. The use of modern molecular biology techniques to assess genetic diversity of meiofauna in contaminated vs. uncontaminated sediments is a promising avenue for future research.
Sampling and processing sandy meiofauna
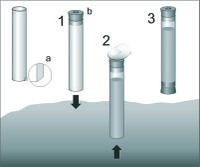
Meiofauna is usually collected from sediment cores, obtained from a perplex tubes of 3,6 cm diameter (surface ~10 cm2 is appropriate for all types of sediment), inserted 15 cm deep into the seabed (Figure 7). From each site, six (at least three) replicates are taken. Sediment is gently pushed out from the tube and cut into the slices. The slices are usually taken from particular layers: 0-1, 1-2, 2-3, 3-4, 4-5, 5-10 and 10-15 cm. The core is sometimes cut every 1 cm. Each sediment slice (subsample) is placed into the separate jar, fixed with 4 % neutral formaldehyde solution and stained with Bengal Rose, to obtain a pink/reddish colour of the sample. For extraction of meiofauna from sediment, two methods are used depending of the amounts of the detritus or silt-clay in the sediment. The decantation method is used when the sediment is a sand with low amounts of detritus or silt-clay. In the laboratory, each slice is placed in the 1000 ml cylinder, filled with tap water and shaken vigorously, to suspend the sediment grains. The water is then filtered through 0.030 mm screen, and the procedure of shaking and flotation is repeated 10 times. All meiofauna organisms are retained on the screen, are gently washed into the Petri dish with measuring grid on the bottom and counted under the low power stereo microscope. The extraction of meiofauna from mud or detritus is done most efficiently in a centrifuge based on a density gradient principle[12]. In this method, liquid must be used with a density larger than the density of meiofaunal organisms (Ludox, density of 1.15). The method consists of placing a sediment sample in the Ludox solution and centrifuging it at 1800 g for 10 minutes. The meiofauna organisms are retained in the 'gel' once the sediment is at the end of the tube. The centrifugation is repeated three more times. This method does not work for heavy foraminifera, because their density is often equal to the sediment grain. In the laboratory, the meiofauna organisms on the measuring grid are counted and identified.
Latitudinal distribution of meiofauna
Meiofaunal samples from arctic (Bear Island, Franz Josef Land, Hopen, Kolguev), temperate (Baltic Sea, North Sea), subtropical (Tunisia, Greece), tropical (Emirates, Ghana) and antarctic sandy beaches were collected at the medium water mark (Kotwicki et. al 2005[13]). The highest average meiofaunal density was found in the temperate zone (1300 individuals per 10 cm2) and the lowest in both polar regions: in arctic (79 individuals per 10 cm2) and in antarctic (35 individuals per 10 cm2) samples. The results of the classification illustrate the clear difference between the polar sampling sites on the one hand and the more temperate beaches on the other hand. Nematodes dominated the meiofauna community in warm regions, while turbellarians were more common in cold water regions. Sixteen higher taxa were recorded in tropical sites, while only eight taxa were observed in the sampled cold regions. This difference was mainly due to the presence of small specimens of macrofauna in the tropics. When only ‘true meiofauna’ higher taxa were compared, no latitudinal trends were found.
In general, high meiofauna density can be found in intertidal muddy estuarine habitats, while much lower values are recorded in the deep sea [14]. In fine sediments such as organic rich muds, meiofauna densities of 104 individuals per 10 cm2 and more are common [15]. The available meiofauna data show a large within-site (within-region) variation in the temperate zone while there was very little variation within the meiofauna densities of the antarctic and arctic zones[13]. These patterns demonstrate that attempts to estimate global biodiversity from the results of regionally based studies must include the significant variation in diversity among sampling sites. The salinity effect on meiofauna occurrence was not clear – two brackish water locations have the same range of meiofauna density as full marine sites[13]. As was reported in cited literature, the lower salinity was not associated with decrease of meiofauna[16].
In terms of taxonomical composition, the meiofauna taxa that were encountered during the study of Kotwicki et. al (2005)[13] are similar to those of muddy sediments. There were no meiofauna taxa found that were restricted to shallow permeable sediments only. The percentage composition, on the other hand, differed significantly between the different study sites along the latitudinal gradient. Nematodes comprised in general more than half of the total meiofauna abundance except in both polar regions (arctic and antarctic), where turbellarians were the dominant meiofauna group.
Some small macrofaunal crustacean species (Cumacea, Amphipoda, Mysidacea) that can occasionally be found in meiofauna samples, were absent in the littoral zone of polar waters[13]. Small size in macrofauna is often associated with a fast development, r-strategy and warm environmental conditions that permit fast egg incubation and growth[17]. That strategy is unlikely to be fruitful in cold regions. These results support the hypothesis that warm regions support fast growing, smaller and more abundant organisms, and cold regions are dominated by larger and less abundant meiofauna.
Macrofauna taxa may contribute to the meiofauna size class only in the tropics. A lower number of taxa was collected in both polar sites. In combination with slightly higher diversity in the temperate and tropic zones, this supported the general pattern of diversity increase towards lower latitude. When only ‘meiofauna sensu stricto’ (i.e., without the small macrofaunal organisms) were taken into account, no clear latitudinal change could be found. Data at species level can give a more detailed and perhaps different outcome. Archambault and Bourget (1996)[18] showed that large-scale heterogeneity explains a larger proportion of the variance in macrofauna species richness than substratum heterogeneity on a more local scale.
In this context, it is reasonable to refer to the important human pressure on temperate beaches. Investigations of the effects of recreational pressure (trampling, beach cleaning and nourishment) suggest that a highly diverse meiofauna and diatom assemblage in undisturbed beaches may act as an effective biological filter for some types of pollutant, while less diverse, but more abundant biota in disturbed areas are more effective in processing organic matter (self-cleaning of the beach)[19] [20]). Largely as a result of conflicting uses of coastal habitats, losses of marine diversity are highest in coastal areas. The best way to conserve marine diversity is to conserve habitat and landscape diversity in the coastal area. Marine protected areas are only a part of the conservation strategy needed. A framework for coastal conservation should include integrated coastal area management, where one of the primary goals is sustainable use of coastal biodiversity[19].
Related articles
References
- ↑ Huettel, M. and Boudreau, B. 2000, Transport and reaction in permeable sediments. Coastal Ocean Processes Newsletter Issue 11: 3-5
- ↑ Huettel, M. et al 2002,Understanding the biocatalytical sand filter in the shelf. LOICZ Newsletter 25: 1-4
- ↑ Huettel, M. et al 2003, Coastal sands as biocatalytical filters (COSA. Coastline 12(1): 8-11
- ↑ 4.0 4.1 Giere, O. 2009, Meiobenthology: The microscopic fauna in aquatic sediments. Second Edition, Berlin: Springer Verlag
- ↑ Angel M.V. 1994. Spatial distribution of marine organisms: patterns and processes. In: Edwards P.J., May R.M. and Webb N.R. (eds) Large-Scale Ecology and Conservation Biology. Oxford University Press, Oxford, UK, pp. 59–109
- ↑ Boudreau B.P., Huettel M., Froster S., Jahnke R.A., McLachlan A., Middelburg J.J. et al. 2001. Permeable marine sediments: overturning an old paradigm. EOS 82: 133–136.
- ↑ Armonies, W. and Reise, K., 2000, Faunal diversity across a sandy shore. Mar. Ecol. Prog. Ser. 196: 49-57
- ↑ McLachlan, A. and Brown, A.C. 2006, The ecology of sandy shore. Second Edition, Amsterdam: Elsevier
- ↑ McIntyre, A.D. 1969, Ecology of marine meiobenthos. Biol. Rev. 44: 245-249
- ↑ Jahnke, R.A., Nelson, J.R., Marinelli, R.L. and Eckman, J.E. 2000, Benthic flux of biogenic elements on the Southeastern US continental shelf: influence of pore water advective transport and benthic microalgae. Continental Shelf Research 20(1): 109-127
- ↑ Shum, K.T. and Sundby, B. 1996, Organic matter processing in continental shelf sediments - The subtidal pump revisited. Mar. Chem. 53(1-2): 81-87
- ↑ Heip, C., Vincx, M., Vranken, G., 1985, The ecology of marine nematodes. Oceanogr. Mar. Biol. Ann. Rev. 23: 399-489
- ↑ 13.0 13.1 13.2 13.3 13.4 Kotwicki, L., Szymelfenig, M., De Troch, M., Urban-Malinga, B. and Weslawski, J.M. 2005. Latitudinal biodiversity pattern of meiofauna from sandy littoral beaches. Biodiversity and Conservation 14: 461–474.
- ↑ Coull B.C. 1988. 3. Ecology of the marine meiofauna. In: Higgins R.P. and Thiel H. (eds) Introduction to the Study of Meiofauna. Smithsonian Institution Press, Washington, DC, pp. 18–38.
- ↑ Ellison R.L. 1984. Foraminifera and meiofauna on an intertidal mudflat, Cornwall, England: populations; respiration and secondary production; and energy budget. Hydrobiologia 109: 131–148.
- ↑ Brown A.C. and McLachlan A. 1990. Ecology of Sandy Shores. Elsevier, Amsterdam, The Netherlands, 328 pp.
- ↑ Steele D.H. and Steele V.J. 1986. The cost of reproduction in the amphipod Gammarus lawrencianus Bousfield. Crustaceana 51: 176–182.
- ↑ Archambault P. and Bourget E. 1996. Scales of coastal heterogeneity and benthic intertidal species richness, diversity and abundance. Marine Ecology Progress Series 136: 111–121.
- ↑ 19.0 19.1 Weslawski J.M., Urban-Malinga B., Kotwicki L., Opalinski K., Szymelfenig M. and Dutkowski M. 2000. Sandy coastlines – are there conflicts between recreation and natural values? Oceanological Studies 24: 5–18.
- ↑ Gheskiere, unpublished data
Please note that others may also have edited the contents of this article.
|