Shore protection vegetation
This article provides an overview of plant species that contribute to strengthening the shore protection function of dune coasts. The focus is on plants that can cope with the saline, nutrient-poor and harsh hydro-sedimentary conditions in the zone between the high-water (HW) line and the more stable backshore. These are species that can grow and thrive in shifting sands, that have a strong sand-binding capacity due to an extensive root system and that favor sand accumulation with their foliage.
The overview has not the pretention to be exhaustive. There are hundreds of plant species occurring along the shores of different continents that are adapted to nearshore conditions. The inventory (Table 1) focuses on species with a high shore protection capacity and that are common to shores worldwide. Shore vegetation with a low sand-binding capacity is not included. The selection is probably biased towards species that are well described in the literature and may ignore some important less common and less-studied species.
Contents
Shore protection function
Shore vegetation contributes to shore protection in two complementary ways (Feagin et al., 2015[1]; Bryant et al., 2019[2]):
- by decreasing wave runup, due to the frictional effect of stems and foliage;
- by decreasing sand loss by wave backwash, due to the effect of plant roots on sand aggregation and fixation.
These functions increase the retention of sand volume of the upper beach and backshore during storms and high water levels. This sand also nourishes further aeolian inshore transport and increases the sand volume of the dunes behind.
Many beach and foredune vegetation species thrive under regular, moderate sand burial. This holds in particular for marram grass (Ammophila), which therefore develops deep root systems (Brown and Zinnert, 2018[3]). These root systems, especially dense fine root structures, contribute more to erosion control than the above-ground biomass (Figlus et al., 2022[4]; de Battisti and Griffin, 2020[5]). The sand-binding function of vegetation is not only because roots retain the sand, but also because roots improve the soil structure, by adding organic material (due to plant turnover and leaf litter), moisture (due to surface shading by plants), and by strengthening the sand aggregation function of microorganisms and fungi[1]. Experiments and field observations show that vegetation increases the stability of dunes during wave attack (Silva et al., 2016[6]). However, vegetation is likely not strong enough to prevent beach and dune erosion under extreme storm conditions. Laboratory experiments even suggest that vegetation may exacerbate erosion under certain severe conditions (Feagin et al., 2023[7]). The strength of the dune belt to withstand severe storm conditions depends primarily on the sand mass of the entire dune complex rather than on the vegetation. The sand mass should be large enough to prevent breach of the dune belt and protect the hinterland against flooding (see Dune erosion). However, the presence of vegetation, the vegetation density, species present, and density and depth of the roots all assist in reducing the degree of scarping and dune erosion (Davidson-Arnott et al., 2020[8]).
Recolonization of eroded parts of the beach, dune scarps and foredunes by vegetation stimulates beach restoration, although ongoing erosion of exposed sediment-deprived beaches will not be stopped[1]. Post-storm beach restoration also requires sand supply from the surf zone to the beach by hydrodynamic processes, in particular wave-induced onshore sand transport and longshore drift (see Shoreline retreat and recovery). Deficient natural onshore sand transport can be remedied with artificial sand nourishment. Field observations show that beach erosion will induce dune retreat while beach accretion will stimulate dune progradation (Davidson-Arnott et al., 2018[9]).
No important difference in vegetation type is observed between prograding and retreating coasts (Konlechner et al., 2019[10]), but rapidly retreating coasts do not display the typical vegetation community succession patterns (Bitton and Hesp, 2013[11]), and on some eroding coasts the final successional vegetation community may be adjacent to the shoreline.
Adaptations
In order to withstand the harsh conditions prevailing on the open shore, species have developed several specific physiological traits. Typical are the following adaptations (Hesp, 1991[12]; Du and Hesp, 2020[13]).
- to salinity and salt spray: succulence, leaf wax, thick cuticles, osmotic adaptation and glands that exude salt. It should be noted that for beach and foredune vegetation, salt spray is not only a stressor but also an important supplier of nutrients[14]
- to drought: succulence, hairy leaves, leave rolling, uptake of dew, roots that reach to the water table and stomata that can be closed
- to sand burial: increased development of roots, rhizomes, stolons and shoots, increased seed production, germination strategy; survivorship of seedlings and adult plants under sand burial is a major selective force in the evolution of dune vegetation (Maun, 1998[15])
- to nutrient deficiency: root nodules that produce nitrogen compounds, uptake of P and N from rhizosphere bacteria and fungal activity in the soil
- to washing away by swash: rapid recolonization through efficient dispersal of seeds by wind and currents, germination of seeds delayed until conditions are favorable (dormancy)
All species that thrive on the backshore have at least some of these adaptations. However, some species are better adapted than others. When species are displaced from their regions of origin to other regions, native species can be outcompeted. This has consequences for the biodiversity as well as for the morphology of the dune landscape (Wiedemann et al., 2003[16]; Doody, 2013[17]; Hilton et al., 2009[18]).
Habitats
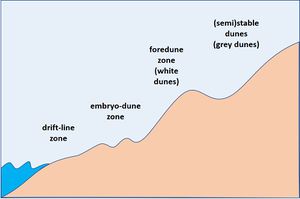
The exchange of sand between sea and land takes place in the shore zone above the mean HW line up to the foredune that is attacked by waves under heavy storm conditions. Most important for shore protection are therefore species that bind and accumulate sand in this zone; and the corresponding habitats are defined in the European Habitats Directive (Natura2000) as (see Fig. 1):
- - Annual vegetation of drift lines (1210)
- - Embryonic shifting dunes (2110)
- - Shifting dunes along the shoreline with Ammophila arenaria (2120)
The latter zone is often designated as 'foredune zone', 'yellow dunes' or 'white dunes'. An overview of common species occurring in these habitats is presented in Table 1. Only species with a sand-binding or dune-building function are considered.
Drift-line zone
The drift line is the location where organic floating debris and wrack (mainly remains of sea grasses and macroalgae) is deposited by waves running up the beach. In reality it is not a line but a zone around the maximum wave run-up, which is shifting with meteorological conditions and with the spring-neap tidal cycle. Most of the species in this zone are so-called pioneers. In fact, they are not really the primary colonizers, because their settlement is facilitated by micro-organisms and fungi, who aggregate sand particles in small clumps in which moist and nutrients are incorporated (McLachlan et al., 2018[19]). Symbiose of plant roots with vesicular–arbuscular mychorrhizal fungi is crucial to the development of pioneer species (Maun, 1994[20]). A special adaptation of drift-line species is their ability to feed on material - litter and wrack - washed ashore by waves. For typical drift-line species, such as Cakile, development is strongly enhanced by the presence of buried organic material (Del Vecchio et al., 2017[21]; Van Egmond et al., 2017[22]). Plants are adapted to the high mobility of the substrate by an extensive root system. However, they generally do not resist storm waves and are therefore short living. Survival of the species is ensured by growing above the high spring tide line on the highest portion of the backshore, a high production rate, and efficient spreading and germination of seeds. Listed species (Table 1) commonly found in different parts of the world near the drift line include the genera: Cakile, Honckenya, Salsola, Sevusium, Spinifex, Uniola , Panicum, Calystegia, Canavalia, Atriplex, Medicago, Scaevola, Carex, Glaucum, Eryngium, Ipomoea, Thinopyrum, Traganum, Elymus.
Embryo-dune zone
Dune formation starts high up on the dry beach or upper backshore. The development of embryo dunes (also known as incipient foredunes) is mainly due to plants with erect stems that are capable of capturing sand that is blown by the wind over the beach. The sand accumulation around an individual plant or a group of plants is called a nebkha. Merger of nebkhas eventually leads to the formation of a continuous shore-parallel incipient foredune. Nebkha-accumulating plants are mostly grasses such as Elymus, Leymus, Ammophilla (in cold/temperate climates), Spinifex, Uniola, Panicum, Carex (in warm climates). Elymus, Leymus and Spinifex are primary colonizers, whose growth is stimulated by sand burial. They are partially replaced by species that colonize the area after the development of an initial dune. Embryo-dune formation is also initiated by plants such as Acacia, Tamarix, Atriplex and Traganum (warm to hot, and arid climates). Note that dunes also occur on the margins of lakes and ponds, and so Willows and other species may constitute the pioneer species on lake margins where the adjacent water body comprises fresh water (e.g. for the Great Lakes[9]). Embryo dunes are further stabilized and developed by smaller plants that form dense communities. Species of the genera Honckenya, Sporobulus, Anthemis, Carpobrotus, Medicago, Eryngium, Zygophyllum may be widespread on embryo dunes.
Foredune zone
Foredune formation results from ecogeomorphic interactions between plants, relief and aeolian and coastal processes (waves, overwash) (Hesp and Smyth, 2019[23]). The foredunes (a.k.a. 'established foredunes') of non-vegetated coasts generally have a gentle slope, while vegetated coasts have a steeper foredune slope[19]. This holds in particular for dunes vegetated with Ammophila, which is a very effective sand binder and dune builder that prevents downslope sand transport even on steep slopes. Ammophila arenaria and Ammophila breviligulata are European and American beach grasses that have been introduced in many regions worldwide. In some regions, Ammophila arenaria can be an aggressive invader, for example in California, where it replaces the native foredune vegetation, greatly reducing species diversity[24][25]. Many other species also contribute to the stabilization and further development of foredunes by forming a dense vegetation cover. Sand-binding species common to foredunes in several regions around the world include the genera: Eryngium, Euphorbia, Carpobrotus, Calystegia, Acacia, Westringia, Oenothera, Anthemis, Ficinia, Paspalum, Casuarina, Medicago, Zygophyllum.
Muddy shores
In temperate climate zones, muddy shores occur mainly in sheltered areas within bays, lagoons and estuaries. The dominant vegetation species are Sporobulus and Spartina, which stabilize mudflats and contribute to their transformation into salt marshes, see Dynamics, threats and management of salt marshes. In subtropical and tropical climate zones, muddy shores occur also on the open ocean. An important coastal protection function is accomplished by mangroves that attenuate waves and currents and favor sedimentation, see Mangroves. Protection against erosion by waves and currents can also be provided by Chrysopogon (Vertiver grass), that binds soil with very large deep roots. Vertiver grass is planted in many subtropical and tropical countries along water courses for the protection of shore and river banks.
Climate zones
Dune coasts are present along coasts worldwide, in all climate zones, see Fig. 2. Each climate zone has its own typical vegetation. Some vegetation species have a wide climate tolerance, but species that are specifically adapted to certain climate conditions generally prevail (Doing, 1985[27]). Species that thrive under a broad range of conditions are species of the genera Ammophila, Cakile, Arctotheca, Salsola, Calistegia. In temperate and Mediterranean climates the dominant dune-building species is Ammophila arenaria (marram grass); a similar species exists in N. America, Ammophila breviligulata. In warmer climate zones (southern US, Mexico, Caribbean) A. Brevigulata is replaced by Uniola paniculata. In colder climates A. arenaria is replaced by Elymus farctus and Leymus arenarius. The major dune-building species in East Asia is Carex kobomigi, which has become an invasive species in the US, and in the Australasian region Spinifex is the dominant pioneer species, although this is replaced by Canavalia and Ipomoea in more tropical regions, and in Asia.
In temperate climatic zones, wind and sand-catching grasses produce dunes with a stronger relief than dunes in warm climatic zones. On subtropical and tropical sandy coasts Spinifex littoreus and Scaevola plumieri contribute to dune-building, but creeping vegetation of the genera Ipomoea and Canavalia is often dominant in more tropical areas (Hesp, 2003[28]).
In arid climates, Tamarix and Traganum can stabilize shifting sands by creating nebkhas that develop into hummocky dunes (Hernandez-Cordero et al., 2018[29]).
Dune management
Along many shores, dunes have a crucial function for protecting coastal settlements (or the low-lying hinterland) from flooding in case of extreme storm surges. If this protection is not sufficient, hard protection structures can be necessary to raise the protection function to the required level. However, hard protection structures modify the sand distribution along the coast, creating or aggravating erosion (see for example Human causes of coastal erosion and other Coastal Wiki articles on hard structures). Artificial sand nourishment avoids this problem, but is often a costly solution. Another option is to strengthen a coastal-defense dune through the incorporation of a hard concrete or rocky core (Oderiz et al., 2020[30]). The need for engineering interventions can be reduced – generally not excluded, however – by stimulating natural dune building processes. This can be done with sand-trapping fences; fences stimulate dune growth in height, but not necessarily in volume (Nordstrom et al., 2012[31]; Li and Sherman, 2015[32]; Itzkin et al., 2020[33]).
Compared to other options, dune reinforcement by stimulating natural vegetation growth or planting has many advantages: it is relatively inexpensive to implement and maintain, it offers the best resilience and it strengthens natural and scenic values, and ecosystem functioning[1]. A simple measure that stimulates the formation of incipient dunes is not to remove beach wrack; evidence from the field suggests that this will increase the number of incipient dunes and the size of swales. Topographic variability will be enhanced and the number and types of habitats for flora and fauna increased[31].
Planting of sand-trapping vegetation stimulates dune growth, through increased net transfer of sand from the bare beach in front of the plantation. Dense vegetation promotes the development of high narrow dunes[34]. Dune growth can further be stimulated by planting species with broad upright stems in staggered formation to the prevailing wind direction (Charbonneau et al., 2021[35]). The total volume of trapped sand depends in the first place on the rate of wind-blown transport to the dune and on the total biomass and characteristics of the plants. The effectiveness of the vegetation decreases when plant growth cannot outpace the sand accumulation[36]. See also the article Dune development.
Lack of understanding - why certain species may or may not prosper under existing conditions - is an important issue in dune vegetation programs. It is most naturel to use native species in coastal vegetation (or revegetation) programs, and in some countries (EU, US) there are legal regulations in this regard. However, more effective dune-building species might be wanted in some cases (more effective in terms of sand fixation, dune-building, soil improvement, adaptation to local conditions, etc.). Introducing non-native species has not always been successful; the species may not thrive, while in other cases the species propagation becomes invasive and alters the entire dune flora (e.g. Ammophila introduction in California and New Zealand; Thinopyrum introduction in South Australia; Acacia introduction in South Africa [16]). The success of vegetation programs using native species is equally uncertain (Hanley et al., 2014[37]). Before introduction of new species, one should find out why endemic species are not there and remove any obstacles that may be responsible for this. Experimental testing should be part of the vegetation programs. In general, introduction of a new species should be avoided.
The causes for the degradation of dune vegetation are multiple. Common causes are[17]:
- loss of dune surface area (urbanization, conversion to agriculture, shoreline retreat);
- soil degradation (e.g. eutrophication, mainly through atmospheric deposition of nitrogen);
- changes in the groundwater table (e.g. water extraction; see also Beach groundwater);
- disturbance (trampling by visitors, sand mining).
Examples are given in the articles on sand dunes in European countries, see Category:Sand dunes. In several countries (European Union, some states in the US) legal prescriptions have been issued for the protection of dune ecosystems. Many other countries have defined setback areas (see Setback area), but enforcement is often inadequate. Monitoring the status of the dune vegetation is essential to detect deterioration in time, to find out what the causes are and to take adequate dune management measures.
| Name (popular name) | Shore protection | Regions | Environmental conditions | Soil type | Propagation | Planting | Other properties |
|---|---|---|---|---|---|---|---|
| Acacia longifolia subsp. sophorae (Coastal Wattle) | Pioneer species in coastal sand dunes. Shrub, 0.5-3 m tall, 10-15 m wide. | Native to South Australia. Introduced to New Zealand, South Africa, Spain and Portugal. | Salt tolerant. | Various types of mobile sandy soils. | By seeds or vegetatively (branches root when touching the ground). | Can be established from seedlings or by direct seeding (pre-soaked for 12 hours in warm water). | Fixes nitrogen. Most Acacia plants die after 8-10 years. Aggressive invasive species, alters indigenous vegetation. |
| Ammophila arenaria (Marram grass, European beach grass) | Pioneer species with great sand-binding and sand-trapping capacity. Initiates foredune growth on flat beaches. Roots extend one to several meters deep. Stimulates the development of high/steep foredunes[25]. | Native to southern/western Europe. Introduced: N. America, S. Africa and Australia. A similar species Ammophila breviligulata (American beach grass) is native to N. America. | Resistant to frost and temperatures up to 50ºC. Tolerates moderate salt spray, drought and low water table. | Well-drained, poor, mobile sandy soils, calcareous preferred. Growth stimulated by sand burial (<1 m/year); Ammophila declines without active sand deposition. | Rhizomes spreading horizontally and vertically. Establishment from seed is less common. | Seedlings in dry sand with moist at about 10 cm below surface. Minimum planting depth about 30 cm. Period: late fall, winter, and early spring months; temperature between 0 and 15 ºC. | Invasive species in some countries, inhibiting growth of native plants. Fixes nitrogen. Ammophila is replaced in stabilized dunes by other species such as Festuca rubra (red fescue grass). |
| Anthemis maritima (Seaside Chamomile) | Pioneer species that contributes to stabilization of mobile dunes, adapted to resist damage from wind-blown sand. | Native to the Mediterranean region, distributed to southwest Asia and W. Africa. | Sunny exposure, salt tolerant. | Lives generally on alkaline well-drained nutrient-poor beach sand, occasionally on sea cliffs and shingle beaches. | Self-pollinating plant. Seeds fall close to the mother plant. | Extraction of essential oil for aromatic and medicinal use. | |
| Arctotheca populifolia (Beach Daisy) | Pioneer species of beach and dunes. Highly effective sand binder that forms hummocks ("nabkha's") by collecting wind-blown sand around it. Often associated with Gazania rigens. | Native to South Africa. Introduced to Australia. Similar species A. calendula introduced in southwestern Europe. | Mediterranean climate, salt tolerant. | Bare sand; variety of soils. | Seeds can be wind-dispersed and also spread by ocean currents. | From seeds or from soft-tip cuttings made in spring and during the growing season. | As an invasive species in Australia, it competes with native plants such as "Spinifex sericeus", by binding sand more efficiently. |
| Cakile Maritima (Searocket) | Pioneer species settling close to the high-water line. Forms low erosion-sensitive hummocky dunes. Single or clumped plants accumulate sand and add organic matter to the soil, thus providing more amenable habitats for the establishment of secondary colonizers. | Cakile maritima native to Europe and N. Africa. Introduced in Australia, New Zealand, Japan. Cakile edentula native to USA. | Resistance to salt spray. | Grows best on moist or wet beaches, alkaline preferred. | Species with seed dormancy. Efficient settling of seedlings, rapid growth, ability to flower under a range of photoperiods. Large numbers of fruits, short life cycle. Seeds can be dispersed by currents. | By seeding or by cuttings. | Invasive species. The plants are sensitive to fungi and are consumed by a variety of phytophagous insects and vertebrates; in response, Cakile manufactures glucosinolates that provides some protection. |
| Calystegia soldanella (Beach Morning Glory, shore bindweed) | Sand-binding pioneer species with a wide-spreading, branching rhizomatous rootstock. Adapted to sand drift, developing best on a buried flood mark and resistant to incidental saltwater flooding | China, Korea, Russia, Africa, Asia, Australia, Europe, North America, Pacific Islands, South America. | Tolerant to salt spray, sand burial and droughty conditions. Sunny exposure, sensitive to frost. | Along sandy shorelines, also in shell banks, fine gravel or pumice. The plant prefers well-drained light moist soils. | Flowers are pollinated by insects; the plant produces large seeds that can be dispersed by seawater. | From seed or rooted plant fragments. | |
| Canavalia maritima (Sea Bean) | Spontaneous sand binder on beaches, cliffs, and dunes. Climber plant that creeps over other coastal plants. Bacteria that form nodules on the roots fix atmospheric nitrogen, made available also to other plants. | South Atlantic and Gulf coast US, Mexico, West Indies, Central America, South America, and the South Asia. | Tropics and subtropics. | Dry well-drained nutrient-poor sandy soils, highly salt tolerant. | Seeds can float considerable distances in the sea, thereby explaining the plants wide distribution. Can also root from nodes at the stems. | Can be grown from seed (soaked in water for several hours to speed up germination.) | Used as drug. |
| Carex kobomugi (Asiatic Sand Sedge) [38] | Dune-stabilizing species, found on the upper parts of ocean beaches, on primary dunes, back-dunes, inter-swale areas. Sand burial stimulates the growth of rhizomes. | Native to China, Japan, Korea, Taiwan. Introduced in northeastern USA. | Temperate climate: Warm average temp. > 10°C, Cold average temp. > 0°C. Wet or dry summer. Grows in semi-shade (light woodland) or full sun. Tolerates maritime exposure. | All types of soil, with preference for moist or wet soil. | Spreads primarily by vegetative means, through production of rhizomes. Long-distance dispersal of salt-tolerant seeds or plant fragments by ocean currents | Replanting of large clumps. Seed-sow in situ in the spring in a moist soil in light shade. | Invasive species, prohibited in several US states. |
| Carex pumila (Dwarf Sand Sedge) [39] | Sand-binding species of the foreshore, cliff base or sea inlets [40]. | Australia, New Zealand, Chile, China, Japan and Korea. | Full sun, salt tolerant. | All types of freely draining sandy or gravely soils. | By rhizomes. It often regenerates naturally from seed spread by freshwater. | Sand sedge can be easily grown from seed and planted along the toe of foredunes near stream mouths. | |
| Carpobrotus glaucescens (Pigface) | Pioneer species growing on the front of sand dunes. Sandbinder allowing more effective sand-trapping species (e.g. Spinifex) to take a hold. If covered with sand the plant can survive, growing upwards and producing a new plant mat over the old one. | Native to Australia. Similar species C. edulis and C. virescens are native to S. Africa and distributed to other continents. | Mediterranean climate. Sunny or semi-shade exposure. Can tolerate drought. Is able to withstand salt spray, strong winds and sand blast. | Any well-drained light (sandy) or medium (loamy) soils. | The species is hermaphrodite (has both male and female organs). Propagates by seeds and by roots that develop at nodes along the stems. | Can be grown either from seed or from cuttings. | |
| Casuarina equisetifolia (Australian pine tree, Filao) | Tree, stabilizer of upper beach and coastal sand dunes, by forming dense stands on coastal foreshores. | The native range extends throughout Southeast Asia, Northern Australia and the Pacific Islands. Introduced to the southern USA, West and South Africa, India, southern China and Brazil. | Tropical (subtropical) climate. Tolerant to extreme environmental conditions such as drought, low nutrient availability, salt and salt spray and burial by sand. | Tolerates calcareous soils, drought, granitic soils, poor soil and waterlogging. | Casuarina propagates naturally by seed. | By direct seeding or with seedlings elevated in a nursery. Can also be propagated vegetatively by cuttings (shoots). | Highly competent invader; colonizes newly disturbed and nutrient-poor sites because of their high fecundity, protracted reproductive season, wide seed dispersal, and tendency to form monospecific stands. Fire-sensitive. |
| Chrysopogon zizanioides (also Vetiveria zizanioides, Vertiver grass) | Plants used to stabilize stream banks and to provide protection against erosion by currents and waves. Also used to block water runoff, to increase water infiltration. Vertiver stimulates sediment deposition; as silt builds up behind a vetiver plant new roots grow out of buried nodes to match the new level of the soil surface. | Origin: South India. Distributed to tropical countries around the world. | Tropical, subtropical climate. Full sun exposure. Temperature in the range -15 ºC to +55 ºC; optimum growth at 25 ºC. Medium to high annual precipitation. Salt tolerant. | Wet sandy loam soils preferred. Drought tolerant due its deep roots (3-4 m). Accepts large range of soil pH. | Neither stolons nor rhizomes. Propagated by clump subdivision. | Soil stabilization by planting one or several hedgerows, forming a long-lasting terrace where sediment is trapped. Low-cost, labor-intensive technique for civil works protection, claimed to have a high benefit/cost ratio. | Non-fertile, non-invasive. Not strongly affected by pests and diseases, not acting as host for pests or diseases that might attack crop or garden plants. The roots are used for many purposes. Vertiver oil is used in oriental fragrances. |
| Elymus farctus subsp. boreoatlantica / Elytrigia juncea / Agropyron junceum (Sand Couch-grass) | Sand-trapping pioneer species; initiates the formation of embryo dunes but rarely a new dune ridge. Can accommodate considerable ongoing accretion by upward grow; partial recovery after temporary burying[41]. | Native to Northern and Western Europe and temperate Asia. The closely related species Thinopyrum junceiforme was introduced to Australia. | Cold and temperate climate. | Grows on low embryo dunes (less frequently on white dunes), on a sandy substrate which is rich in chlorides, with a neutral or alkaline pH. | By seeds and by rhizomes. | Suffers from recreational use of beaches. | |
| Eryngium maritimum (Sea Holly) [42] | Pioneer species of the upper beach, growing on low mobile dunes and binding sand with roots up to 5 m long. High sand accumulation (> 0.2 m/year) is unfavorable for development. Grows typically on sand and shingle beaches, foredunes and yellow dunes. | Native to Europe and adjacent parts of northern Africa and Middle East (shores of the Atlantic Ocean, the Baltic, the Mediterranean, and Black and Azov Seas). Introduced into parts of eastern North America and to Australia. | Mediterranean–
Atlantic climate, mild winters. Grows on open spaces in full sun. |
Well-drained poor sandy soil (low silt/clay content), slightly enriched with nutrients from sea spray, carbonates from shells and material blown from the driftline. | Vegetative reproduction by offshoots from rhizomes or root fragments, dispersed by the sea over short-distances. In Mediterranean regions, reproduction mainly through seeds. | Vegetative reproduction is used in horticulture, where root cuttings are taken in winter. | Eryngium maritimum is unable to withstand competition from faster and more densely growing plant species (e.g., Carex arenaria) and suffers from recreational use of beaches. |
| Euphorbia paralias (Sea Spurge) | Highly efficient sand binder anchored by a deep taproot with several lateral roots. Colonizes bare sand and native dune vegetation, from the high water mark into the dunes, grows through accumulating sand on beach fronts. Also found on rocky foreshores, steep back dunes and mouths of coastal lakes and estuaries. | Native to many countries of Europe, northern Africa, and western Asia. Introduced to Australia. | Temperate and semi-arid regions. Sunny exposure. Tolerant to salt and drought. | Bare, well-drained organic-poor sandy soil. Withstands moderate burial. | Produces large numbers of buoyant salt-tolerant seeds which survive a number of years dispersed on ocean currents. Spreads rapidly once established. Propagation also by root fragments. | Invasive environmental weed in Australia, reducing floral and structural diversity. Toxic: sap in the leaves and stem is irritant and posionous. Euphorbia terracina has characteristics similar to "E. paralias". | |
| Ficinia spiralis / Desmoschoenus spiralis (Pingao, Golden Sand Sedge) | Good sand-binding properties, grows best in the mobile sand at the front of the dune. Partial sand trapping due to the density of its foliage and morphology. Creates active sand dunes (allowing some sand movement). | New Zealand. | Salt tolerant. | Moderate sand movement promotes growth, excessive accumulation or erosion causes dieback. Well-established stands can be excavated by high persistent winds and may die back leaving exposed rhizomes and roots. | By stolons and seeds. | Can be reproduced from seeds or vegetatively from stolons. | Suffers from competition with introduced marram grass and animal grazing. Leaves are used as weaving material. |
| Honckenya peploides (Sea Wort grass) | Coastal pioneer plant and sand binder, close to the tidemark and on embryo dunes. Forms mats on sand beaches and gravel beaches. Long-branched rootstocks grow quickly under the sand; finely-branched shoot roots anchor the plant firmly in the sand. A plant that is buried in a storm can send new shoots back up to the surface without having to produce new shoots. | Coasts of temperate and arctic regions of Eurasia, including Britain, and N. America. | Sunny, maritime exposure. Tolerant to frost and to short periods of immersion in salt water. | Light (sandy) and medium (loamy) well-drained dry or moist soils, can grow in nutritionally poor soil. Suitable pH: acid, neutral and basic (alkaline) soils. | Hermaphrodite species, pollinated by insects, wind and wind-blown sand. The plant is self-fertile. | The rootstock produces an abundance of buds from which new shoots grow. | Sea sandwort stabilizes and fertilizes the land, thereby helping more demanding sea-shore plants. In time it changes the conditions in a way that it eventually finds itself in retreat from the ever-increasing competition. |
| Ipomoea imperati (Beach Morningglory, Fiddle-leaf Morningglory) | Colonizer species on the frontal dunes; binds shifting sands by forming mats. Used for dune restoration. Ipomoea pes-caprae has similar characteristics. | Ipomoea imperati occurs on all continents. Ipomoea pes-caprae is a common species of coasts around the Indian ocean. | Tropical and subtropical regions. Drought and salt water tolerant; cannot withstand frequent inundations. | Moist, well-drained, nutrient-poor sandy soils, without humus. Grows in unconsolidated substrate (upper beach), also on the high marsh. | Rapid propagation with vines that spread up to 10 m and roots at the nodes (points where leaves arise). Seed dispersal by flowing in seawater. | Growth from cuttings. Seeds of railroad vine are physically dormant and must be scarified in order to germinate. | |
| Leymus arenarius (Sand Ryegrass, Sea Lyme grass, Lyme grass) [43] | Pioneer species on beach, embryo dunes and foredunes; dune-building grass, stabilizing drifting sands and eroding fronts. Thrives on sand deposition; replaced by other species when sand deposition ceases. Strong similarities with Elymus farctus. | Native to the coasts of northern and western Europe and Iceland. Closely related to Leymus mollis of the northern coasts of North America. | Prefers cool climate but tolerant of hot weather. Highly salt tolerant. Sunny exposure or half-shade. | Grows on low embryo dunes, on a sandy substrate , loam (silt) or clay. | By rhizomes. | Sowing of seeds in sheltered fertilized areas. | Takes up nitrogen into its root system. Changes soil conditions and microclimate, which become more suitable for later successional species. Invasive species in some countries. |
| Medicago marina (Coastal Medick, Sea Medick)[44] | Sand-binding pioneer species with an extensive root system that settles in loose sand close to the sea. | Native to the Mediterranean region, distributed worldwide. | Sunny exposure. Is tolerant of wind and salt spray and can survive severe drought. | It will grow on most soils, even very poor and polluted soil. | Propagation by rhizomes . Self- and cross-pollinating plant. Seeds fall close to the mother plant; dispersal by wind. | The roots have nodules containing bacteria that fix nitrogen, available also to other plants that contribute to further dune stabilization. Essential oil is extracted from the dried aerial parts of M. marina. | |
| Oenothera drummondii (Beach Evening Primrose) |
Mat-forming sand stabilizer that appears after the primary colonizers. It can grow in drifting sand, but also in (semi-)stabilized dunes and on rock cliffs. |
Native to northern Mexico and southern USA. Introduced to Australia, New Zealand, South Africa, China, Egypt, Spain. | Mediterranean climate, sunny exposure. Adapted to moderate salinity and sand movement. | Prefers well-drained slightly moist neutral soil, but can cope with dry conditions due to efficient water use. | Spreads readily by seed into dune areas. | From seed or by root division. | Medicinal use for treating sore throat and eye diseases. |
| Panicum amarum (Bitter Panicum, Coastal Panicgrass) | Stabilizer of semi-mobile dunes. Extensive fibrous root and rhizome system holds the sand in place. Tends to build narrow/steep foredunes[45]. | USA South Atlantic and Gulf coast. | Mediterranean climate. Tolerates moderate saline overspray. | Well-drained poor sandy soil. Strongest growth with moderate sand accumulation around the plants. Replaces Ammophila in warmer regions. | By stolons or rhizomes. | Planting of young tillers, with some roots and rhizomes attached, preferably early spring. Should be mixed with other grasses, e.g. American beachgrass and sea oats. | |
| Paspalum vaginatum (Seashore Paspalum) | Excellent shoreline protector; spreads rapidly, forming dense stands that anchor soil particles and dissipate wave energy. Less suited for dune building. | Origin: Americas. Worldwide occurrence. | Tropical and subtropical zones. Tolerance of a wide range of conditions such as drought, saline or recycled water, varying soil pH, extended periods of low light intensity, flooding or extended wet periods. | Best suited to compacted inorganic marsh soils of moderate salinity. | Spreads by its rhizomes and stolons, forming a thick turf. | Nursery-raised plants or vegetatively; on drier sites, pieces of sod or rooted transplants should be used. Bare-rooted rhizomes can be used on wetter sites. It can be planted and grow where other species would not survive. | Good resistance to insects and diseases. |
| Poa macrantha (Dune Bluegrass, Seashore Bluegrass[46]) | Sand-binding grass, with stolons and an extensive, strong rhizomatous root system. Stabilizes blowing and moving sand of foredunes. | Pacific coast of N. America from Alaska to California. Similar species: Poa douglasii in southern California and Poa billardierei (Sand tussock) in New Zealand. | Temperate climate. Sunny exposure. | Well drained sand soil. | By seeds and rhizomes. | Nursery-raised plants from locally collected seed, also by division of plants. | |
| Salsola kali (Common Saltwort) | Pioneer species in the driftline and upper-beach zones. Roots, rhizomes and buried shoots reduce dune erosion under a swash regime[47]. | Native to northern and western Europe, from Norway to Portugal, including northern Russia. Introduced to America, Australia and New Zealand. | S. kali habitats include cool, temperate, desert, steppe, subtropical, arid life zones. It tolerates a very high range of annual rainfall and annual temperatures and saline soils[48]. | Organic-poor soils. Found in disturbed areas, including shingle and sand beaches. | Self-seeding annual plant; seeds remain viable for 6-12 months and are dispersed by wind, small rodents and birds. | Invasive species; where introduced it displaces native plants and competes for space, water and nutrients. | |
| Scaevola plumieri / Scaevola taccada [49] | Sand-binding pioneer plant on beaches (preference for coral sand), sand dunes, sandbanks, tropical atolls, mangroves and seagrape habitats. Tolerates sand burial and contributes to dune building. S. plumieri and S. taccada are similar species. | Scaevola taccada native to South Asia, East Africa, Pacific islands; Scaevola plumieri native to East and South Africa, Ceylon. Introduced to (sub)tropical America south of Florida. | (Sub)tropical monsoon climate. Grows within in the salt spray area. | Well-drained light/medium shallow sandy soils, seasonally waterlogged. Alkaline/neutral pH. | Fast dispersal along the coast line, canal banks, mangroves, and inland shorelines by fruits that can float for up to one year. Plant fragments or stems may be dispersed on vegetation rafts. | The species also grows from cuttings. | Invasive species in Florida and Caribbean region. Occurs often together with Ipomoea. |
| Sesuvium postulacastrum (Shoreline Purslane) | Pioneer sand-colonising plant that grows on the upper beach and seaward slope of the frontal dune or beach ridge. It traps and holds wind-blown sand and tends to form small ridges or mounds. It does not survive complete burial under wind-blown sand. It also grows well in more protected littoral locations. | Native to Africa, Asia, Australia, North America and South America. | Worldwide in tropical and subtropical regions. Full sun exposure, very tolerant of salty conditions and very drought tolerant (established plants). | Well-drained poor sandy soil, sandy clay, coastal limestone and sandstone, tidal flats and salt marshes The plant tolerates acidic and alkaline soils. No irrigation or fertilizer needed. | The thick stems form roots at the nodes. | From herbaceous stem cuttings. | Was used medicinally to treat scurvy (vitamin C deficiency) and is sold in Asia as a vegetable. Also used for desalination and phytoremediation (soil cleaning by bioaccumulation of toxic substances). |
| Spinifex sericeus / Spinifex littoreus | Pioneer species, highly efficient deep-rooting foredune builder. The upright shoots aid in reducing surface wind velocity and accumulating sand with frequent burial of stems and leaves. A spinifex-dominant dune has typically a low-angle dune face without blow-outs. | "Spinifex sericeus": Australia, New Zealand. "Spinifex littoreus": New Guinea, Indonesia, Malaysia, Philippines, Indochina, Indian Subcontinent, China, Japan. | Fast growth in summer, favored by warm conditions. Tolerant to high winds and salt spray. Withstands extreme temperatures and drought. | Establishes in raw sand. Stimulates sand deposition that in return favors growth. | By stolons (growth 5 m/year) and rhizomes. | 1. Seeds buried 10 cm in moist sand, after prior brush matting (high mortality rate). 2. Runners or tip cuttings of 0.5 m long planted in temperate season on a 1 m grid into moist sand at a depth of 25 cm. | Tolerant to swash. |
| Sporobolus pumilus (Salt-meadow Cordgrass) | Shore stabilizing grass once it is firmly settled. Mostly common in marshland, but also in the dunes. | N America Atlantic coast from Newfoundland to Mexico. | Low drought tolerance; high salt tolerance. | Salty, wet habitat: marshland, moist dune sites. Highly adaptable to a range of soil conditions (loam, organic, sandy, clay). | Reproduces both through seed and vegetative rhizomatous roots. | Multi-stemmed transplants from uncrowded nursery stands. Plants 20 cm deep in moist zone. | |
| Sporubolus virginicus (Seashore Dropseed) | Dune stabilizer on wind-eroded shorelines. It has potential for stream bank stabilization and also roadside slope stabilization. | Tropical zones of Africa, eastern Asia, Australasia, the Pacific, Caribbean, North and South America. | Tropical zones. Adapted to low rainfall and high salinity. | Moist, alkaline, nutrient-poor soil. Sandy or muddy seashores (often below high tide mark) and saline marshes. | Reproduces asexually through stolons and rhizomes. | From rhizomes sections planted in a sterile, well drained medium in a greenhouse. | Grazing plant for cattle. |
| Tamarix nilotica (Nile Tamarisk) | Shrub (2-5 m), sand binder for its extensive root system. Wind-blown sand comes to rest at the foot of the shrub, stimulating its growth and gradually creating a hummock. | Southeast Mediterranean region and east Africa. Also in the Nile valley. Other Tamarix species (T. aphylla, etc.) with similar characteristics around the Mediterranean and in the Middle East. | Adapted to arid subtropical climate, salt tolerant, capable to extract moisture from the underlying saline substrate. It tolerates temperatures from -10 to +50°C and frequent droughts. | Wide variety of soils, loamy soil preferred. | Tamarix can spread both in vegetative mode, by roots or submerged stems, and sexually, by seeds. Seeds can be dispersed by wind and by water. | Salt excreted through the glands in the leaves in the form of litter or 'tears' causes soil salinization, reducing the growth of nearby plants [50]. | |
| Traganum moquinii | Early colonizing, sand-binding bush species of foredunes of arid dunefields that interacts with aeolian sedimentary processes, generating nebkhas, shadow dunes and arid parabolic shaped dunes[51]. | Canary Islands, South Morocco, Mauritania and other close archipelagos, like Cape Verde. | Arid subtropical climate. | Shifting sands. Withstands sand burial by rapid vertical and horizontal growth. | Seeds are dispersed by the wind. | ||
| Uniola paniculata (Sea Oats) | Beach/dune grass with good sand-binding and dune-building capacity. Slow initial settlement. Very persistent. Tends to build narrow/steep foredunes[45]. | USA South Atlantic and Gulf coast, Mexico. | Mediterranean, subtropical climate. Once established, low water requirement and resistant to salt spray. | Well-drained poor sandy soil. Growth stimulated by sand deposition. | By rhizomes. | Transplantation from wild stocks or nursery-grown 2-year old plants. Planting similar to Ammophila and Panicum; young plants require moist soil and low salinity. | |
| Zygophyllum fontanesii / Tetraena fontanesii (Sea Grape) | Contributes to beach stability due to a strong root system; retains embryo dunes. | Western Sahara countries, Macaronesia, northeast Africa. | Sunny exposure. Can withstand intense drought. Requires regular salt spray. | Grows best in coarse, calcareous sandy brackish soils on the dunes; also on rocky soils. | By seeds. | Seeds are very tolerant of salt water, but slow to germinate. Propagation by cuttings. |
Further reading
- Martínez M. L. and Psuty N. P. (Eds.) 2003. Coastal Dunes; Ecology and Conservation. Springer, 386 pp.
- Doody, J.P. 2013. Sand Dune Conservation, Management and Restoration. Coastal Research Library, Vol. 4, Springer, 303 pp.
- Hesp, P.A. and Walker, I.J. 2013. Aeolian environments: coastal dunes. In: Shroder, J. (Editor in Chief), Lancaster, N., Sherman, D.J., Baas, A.C.W. (Eds.), Treatise on Geomorphology, vol. 11, Aeolian Geomorphology. Academic Press, San Diego, CA, pp. 109-133.
- Martinez, M.L., Gallego-Fernandez, J.B. and Hesp, P.A. (Editors) 2013. Restoration of Coastal Dunes. Springer, 347pp.
Related articles
- Dune erosion
- Sand Dunes in Europe
- Dune development
- Ecological enhancement of coastal protection structures
- Dune stabilisation
References
- ↑ 1.0 1.1 1.2 1.3 Feagin, R. A., J. Figlus, J. C. Zinnert, J. Sigren, M. L. Martínez, R. Silva, W. K. Smith, D. Cox, D. R. Young, and G. Carter. 2015. Going with the flow or against the grain? The promise of vegetation for protection beaches, dunes, and barrier islands from erosion. Frontiers in Ecology and the Environment 13: 203–210
- ↑ Bryant, D.B., Anderson-Bryant, M., Sharp, J.A., Bell, G.L. and Moore, C. 2019. The response of vegetated dunes to wave attack. Coastal Engineering 152, 103506
- ↑ Brown, J.K. and Zinnert, J.C. 2018. Mechanisms of surviving burial: dune grass interspecific differences drive resource allocation after sand deposition. Ecosphere 9, e02162
- ↑ Figlus, J., Sigren, J.M., Feagin, R.A. and Armitage, A.R. 2022. The unique ability of fine roots to reduce Vegetated Coastal dune erosion during wave collision. Front. Built Environ. 8:904837
- ↑ de Battisti, D. and Griffin, J.N. 2020. Below-ground biomass of plants, with a key contribution of buried shoots, increases foredune resistance to wave swash. Ann. Botany 125: 325–334
- ↑ Silva, R., Martinez, M.L., Oderiz, I., Mendoza, E. and Feagin, R.A. 2016. Response of vegetated dune–beach systems to storm conditions. Coastal Engineering 109: 53–62
- ↑ Feagin, R.A., Innocenti, R.A., Bond, H., Wengrove, M., Huff, T.P., Lomonaco, P., Tsai, B., Puleo, J., Pontiki, M., Figlus, J., Chavez, V. and Silva, R. 2023. Does vegetation accelerate coastal dune erosion during extreme events? Sci. Adv. 9 (24), eadg7135
- ↑ Davidson, S., Hesp, P.A., Miot da Silva, G. 2020. Controls on dune scarping. Progress in Physical Geography. PPG-19-140
- ↑ 9.0 9.1 Davidson-Arnott, R., Hesp, P.A., Ollerhead, J., Walker, I., Bauer, B., Delgado-Fernandez, I. and Smyth, T.A.G. 2018. Sediment budget controls on foredune height: a comparison of simulation model results and field data. Earth Surface Processes and Landforms 43: 1798-1810. doi: 10.1002/esp.4354
- ↑ Konlechner, T.M., Kennedy, D.M., Cousens, R.D. and Woods, J.L.D. 2019. Patterns of early-colonising species on eroding to prograding coasts; implications for foredune plant communities on retreating coastlines. Geomorphology 327: 404–416
- ↑ Bitton, M. and Hesp, P.A. 2013. Vegetation dynamics on eroding to accreting beach-foredune systems, Florida Panhandle. Earth Surface Processes and Landforms 38: 1472-1480
- ↑ Hesp, P.A. 1991. Ecological processes and plant adaptations on coastal dunes. Journal of Arid Environments 21: 165-191
- ↑ Du, J. and Hesp, P.A. 2020. Salt spray distribution and its impact on vegetation zonation on coastal dunes: A review. Estuaries and Coasts.
- ↑ Zunzunegui, M., Esquivias, M.P., Alvarez-Cansino, L. and Gallego-Fernandez, J.B. 2024. Seawater spray as a significant nitrogen source across coastal dune vegetation gradients. Estuarine, Coastal and Shelf Science 309, 108941
- ↑ Maun, M.A. 1998. Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany 76: 713–738. doi: 10.1139/b98-058
- ↑ 16.0 16.1 Wiedemann, A.M. and Pickart, A.J. 2003. Temperate Zone Coastal Dunes. In: (Eds. Martínez M. L. and Psuty N. P.) Coastal Dunes; Ecology and Conservation. Springer
- ↑ 17.0 17.1 Doody, J.P. 2013. Sand Dune Conservation, Management and Restoration. Coastal Research Library, Vol. 4, Springer, 303 pages. Cite error: Invalid
<ref>tag; name "D" defined multiple times with different content - ↑ Hilton, M.J., Woodley, D., Sweeney, C., Konlechner, T. 2009. The development of a prograded foredune barrier following Ammophila arenaria eradication, Doughboy Bay, Stewart Island. Journal of Coastal Research SI 56: 317–321
- ↑ 19.0 19.1 McLachlan, A. and Defeo, O. 2018. Coastal Dune Ecosystems and Dune–Beach Interactions. In The Ecology of Sandy Shores (Third Edition) 309-329. Academic Press
- ↑ Maun, M.A. 1994. Adaptations enhancing survival and establishment of seedlings on coastal dune systems. Vegetatio 111: 59–70
- ↑ Del Vecchio, S., Jucker, T., Carboni, M. and Acosta, A.T.R. 2017. Linking plant communities on land and at sea: the effects of Posidonia oceanica wrack on the structure of dune vegetation. Estuar. Coast. Shelf Sci. 184: 30–36
- ↑ van Egmond, E.M., van Bodegom, P.M., van Hal, J.R., van Logtestijn, R.S.P., Broekman, R.A., Berg, M.P. and Aerts, R. 2017. Growth of pioneer beach plants is strongly driven by buried macroalgal wrack, whereas macroinvertebrates affect plant nutrient dynamics. Journal of Experimental Marine Biology and Ecology 514–515: 87–94
- ↑ Hesp, P.A. and Smyth, T.A.G. 2019. Anchored Dunes. In: I. Livingstone and A. Warren (Eds.), Aeolian Geomorphology: A New Introduction: 157-178. Wiley Blackwell
- ↑ Barbour, M., and Johnson, A.F. 1977. Beach and dune. In Barbour, M. and J. Major (eds.), Terrestrial vegetation of California. John Wiley and Sons, New York. Pages 223-270
- ↑ 25.0 25.1 Pickart, A.J. 2021. Ammophila Invasion ecology and dune restoration on the West Coast of North America. Diversity 13, 629
- ↑ Martínez, M.L., Psuty, N.P. and Lubke, R.A. 2003. A Perspective on Coastal Dunes. In: (Eds. Martínez M. L. and Psuty N. P.) Coastal Dunes; Ecology and Conservation. Springer
- ↑ Doing, H. 1985. Coastal fore-dune zonation and succession in various parts of the world. Vegetatio 61: 65-75
- ↑ Hesp, P.A. 2003. Coastal Dunes in the Tropics and Temperate Regions: Location, Formation, Morphology and Vegetation Processes. In: (Eds. Martínez M. L. and Psuty N. P.) Coastal Dunes; Ecology and Conservation. Springer
- ↑ Hernandez‐Cordero, A. I., Hernandez‐Calvento, L., Hesp, P. A. and Perez‐Chacon, E. 2018. Geomorphological changes in an arid transgressive coastal dune field due to natural processes and human impacts. Earth Surface Processes and Landforms, 43: 2167-2180
- ↑ Oderiz, I., Knochelmann, N., Silva, R., Feagin, R.A., Martínez, M.L. and Mendoza, E. 2020. Reinforcement of vegetated and unvegetated dunes by a rocky core: A viable alternative for dissipating waves and providing protection? Coastal Engineering 158, 103675
- ↑ 31.0 31.1 Nordstrom, K.A., Jackson, N.L., Freestone, A.L., Korotky, K.H. and Puleo, J.A. 2012. Effects of beach raking and sand fences on dune dimensions and morphology. Geomorphology 179: 106–115
- ↑ Li, B. and Sherman, D. J. 2015. Aerodynamics and morphodynamics of sand fences: A review. Aeolian Research 17: 33-48
- ↑ Itzkin, M., Moore, L.J., Ruggiero, P. and Hacker, S.D. 2020. The effect of sand fencing on the morphology of natural dune systems. Geomorphology 352, 106995
- ↑ Hesp, P.A., Dong, Y., Cheng, H and Booth, J.L. 2019. Wind flow and sedimentation in artificial vegetation: Field and wind tunnel experiments. Geomorphology 337: 165–182
- ↑ Charbonneau, B.R., Dohner, S.M., Wnek, J.P., Barber, D., Zarnetske, P. and Casper, B.B. 2021. Vegetation effects on coastal foredune initiation: Wind tunnel experiments and field validation for three dune-building plants. Geomorphology 378, 107594
- ↑ Derijckere, J., Strypsteen, G. and Rauwoens, P. 2023. Early-stage development of an artificial dune with varying plant density and distribution. Geomorphology 437, 108806
- ↑ Hanley, M.E., Hoggart, S.P.G., Simmonds, D.J., Bichot, A., Colangelo, M.A., Bozzeda, F., Heurtefeux, H., Ondiviela, B., Ostrowski, R., Recio, M., Trude, R., Zawadzka-Kahlau, E. and Thompson, R.C. 2014. Shifting sands? Coastal protection by sand banks, beaches, and dunes. Coast. Eng. 87: 136–146
- ↑ Lea, C. 2005. Fact sheet: asiatic sand sedge. National Park Service, Washington, DC https://www.invasive.org/alien/fact/pdf/cako1.pdf
- ↑ de Lange, P.J. 2020. Carex pumila Fact Sheet. New Zealand Plant Conservation Network. https://www.nzpcn.org.nz/flora/species/carex-pumila/
- ↑ Jung, S.H., Kim, A.R., Lim, B.S., Seol, J.W. and Lee, C.S. 2019. Spatial distribution of vegetation along the environmental gradient on the coastal cliff and plateau of Janggi peninsula (Homigot), southeastern Korea. J. Ecology and Environment 43: 14 https://doi.org/10.1186/s41610-019-0110-y
- ↑ Harris, D and Davy, A.J. 1986. Strandline colonization by Elymus farctus in relation to sand mobility and rabbit grazing. Journal of Ecology 74: 1045-1056
- ↑ Isermann, M. and Rooney, P. 2014. Biological Flora of the British Isles: Eryngium maritimum. Journal of Ecology 102: 789–821
- ↑ Greipsson, S. and Davy, A.J. 1994. Leymus arenarius. Characteristics and uses of a dune-building grass. Icel. Agr. Sci. 8: 41–50
- ↑ Small, E. 2011. Alfalfa and relatives: evolution and classification of "Medicago". NRC Research Press, Ottawa, 727 pp.
- ↑ 45.0 45.1 Hacker, S.D., Jay, K.R., Cohn, N., Goldstein, E.B., Hovenga, P.A., Itzkin, M., Moore, L.J., Mostow, R.S., Mullins, E.V. and Ruggiero, P. 2019. Species-Specific Functional Morphology of Four US Atlantic Coast Dune Grasses: Biogeographic Implications for Dune Shape and Coastal Protection. Diversity 11, 82
- ↑ Oakes, A.J. 1990. Ornamental grasses and grasslike plants. Van Nostrand Reinhold publ., New York
- ↑ De Battisti, D, and Griffin, J.N. 2020. Below-ground biomass of plants, with a key contribution of buried shoots, increases foredune resistance to wave swash. Ann Bot. 125: 325-334. doi: 10.1093/aob/mcz125
- ↑ CABI. Invasive Species Compendium, https://www.cabi.org/isc/datasheet/50297
- ↑ CABI 2019. Invasive Species Compendium. www.cabi.org/isc/datasheet/48817
- ↑ Abdelgawad, A.A.M. 2017. Tamarix nilotica (Ehrenb) Bunge: A Review of Phytochemistry and Pharmacology. J. Microb. Biochem. Technol. 9: 544-553. doi: 10.4172/1948-5948.1000340
- ↑ García-Romero, L., Hernández-Cordero, A., Hernández-Calvento, L. and Hesp, P.A. 2017. Long-term analysis of the role of Traganum moquinii plants in the foredune formation of an arid dunefield (Maspalomas, Gran Canaria, Canary Islands). 19th EGU General Assembly, proceedings from the conference held 23-28 April, 2017 in Vienna, Austria., p.1479
Please note that others may also have edited the contents of this article.
|