Marine mammals' health as an indicator of ecosystem health - tools for monitoring
Contents
Introduction
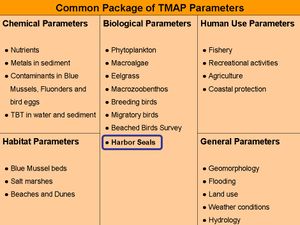
Marine mammals are top predators of the marine food web and can therefore be used as indicators for the ecosystem (Trilateral Monitoring and Assessment Program, TMAP, Fig. 1). Industrial human activities, e.g. fisheries, offshore drilling and wind power generation, as well as pollutants in the environment affect the North and Baltic Sea ecosystems, which are the habitat of marine mammals such as harbour porpoises (Phocoena phocoena), harbour seals (Phoca vitulina) and grey seals (Halichoerus grypus). The present article reports tools to investigate the health status of harbour seals. A dysregulation of the immune system can lead either to suppression or an increased sensitivity (hypersensitivity) of the immune system, which might affect morbidity and mortality in these animals. Parameters of the immune system were investigated as stable biomarkers for disease processes. Furthermore, factors indicating the impact of pollutants were identified and chemically characterised.
Methodology
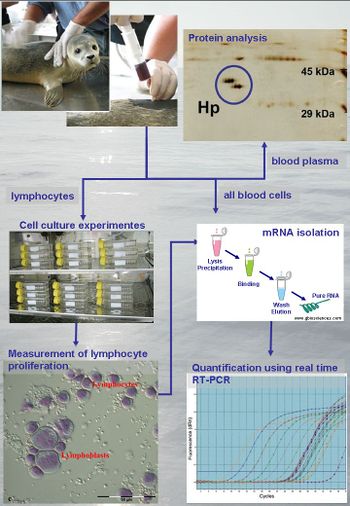
The following parameters of the immune system can be measured in blood samples of captive and free-ranging animals using in-vivo measurements, in-vitro cell culture experiments, and bioanalytical methods (Fig. 2): lymphocyte proliferation, mRNA and protein expression of cytokines and acute phase proteins (APP). Protein structures, and the influence of metal and organic pollutants on these can be analysed and allow, therefore, conclusions about the relevance of the pollutant. Elements indicating the relationship between pollutant burden and health parameters can be analysed in body fluids using mass spectrometry (see article Elemental mass spectrometry).
Measurement of lymphocyte proliferation
Lymphocytes are isolated from blood samples and cultured with and without ions of metal pollutants using a lymphocyte transformation test (LTT, Fig. 2). After an incubation period, transformation and proliferation of the lymphocytes is examined and a stimulation index calculated. Depending on degree of stimulation the reactivity of the lymphocytes and therefore immune system can be detected.
Quantification of cytokine expression
mRNA and protein expression of cytokines and APP can be measured by real time reverse transcriptase polymerase chain reaction (RT-PCR) and mass spectrometry, respectively. Next to quantification of protein concentrations, mass spectrometry allows also the characterization of protein isoforms (Fig. 2). Cytokines as interleukin-1, -2, -4, -6, -10, -12, tumour necrosis factor-alpha, transforming growth factor-beta and APP (e.g. haptoglobin, C-reactive protein, transferrin, metallothionein, heat shock protein) are stimulated by infection, inflammation, trauma or intoxication. They might therefore be helpful to identify an activated immune system and to monitor the health status.
Metal pollution – effects on the immune system
In many areas of the industrialised world pollution by metals is present, which might have an effect on the immune competence of free-ranging marine mammal populations. An imbalance of the immune system caused by pollutants has been suggested to play a role in the incidence of infectious diseases in marine mammals (Jepson et al., 1999[1]; Bennett et al., 2001[2]). Metals affect the function of immune cells by a variety of mechanisms. Depending on the particular metal, its speciation, concentration and bioavailability, permanent metal exposure can result in suppression or stimulation of the immune system.
Metal induced hypersensitivity in seals
A chronic exposure to metal pollutants and the intake via food and water was suspected to cause hypersensitivity reactions in marine mammals. Hypersensitivity reactions were found for Mo > Ni >Ti > Cr, Al > Pb, Be, Sn, (in decreasing frequency as indicated by the present order) in several pinnipeds from the North Sea (Kakuschke, 2006[3]) and a relationship between blood concentrations and hypersensitivity reactions of the particular metals was present (Kakuschke et al., 2005 [4]). Furthermore, an association between lymphocyte proliferation caused by Ni and Be and cytokine expression was detected in a study of a grey seal (Kakuschke et al., 2006[5]). Changes in haematological parameters and liver enzymes in seals with a metal-induced hypersensitivity suggests that hypersensitivity reactions are a complex phenomenon (Kakuschke et al., 2011 [6]). These findings indicate a chronic exposure of marine mammals of the North Sea to metal pollutants.
High susceptibility of the immune system of pups to the toxic effect of metals
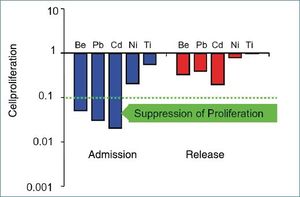
Pups are exposed to metals through transplacental transfer, maternal milk and, at a later stage, contaminated prey. Kakuschke et al. (2008)[7] reported that lymphocytes of new born seal pups are particularly susceptible to the toxic effects of metals, which subsequently decreases with age (Fig. 3). Additionally, the chemical form of the metal compound was shown to play an important role for the immunological effect. Four different mercury compounds were reported to have a suppressive effect on lymphocyte proliferation; however, differences were present between juvenile and adult seals, and depending on the chemical form of mercury. (Kakuschke et al. 2010[8].)
Stress – effects on the immune system
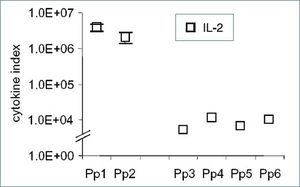
The cytokine expression can be modulated by numerous factors, including stress. Cytokine mRNA expression from two harbour porpoises living in captivity and four accidentally caught wild living porpoises were compared. A stress-induced modulation of the cytokine expression was suspected in the accidentally caught wild animals (Fig. 4, Fonfara et al. 2007 [9] [10]). Furthermore, cytokine and acute phase proteins transcription varied in harbour seal pups during rehabilitation in the seal station Friedrichskoog, Germany, suggesting that these parameters might be useful to assess the health status, maturation of the immune system, and the ability to handle stress in these animals (Fig. 5, Fonfara et al. 2008 [11]).
Acute phase proteins (APPs) – long term studies and protein characterization
Protein blood concentrations of APPs such as haptoglobin (Hp), C-reactive protein (CRP), or transferrin are influenced by several diseases and might, therefore, be useful parameters to monitor health. Long term studies of Hp and CRP levels in harbour seals between 2002 and 2007 showed variations between years, as well as differences between age groups and sex (Kakuschke et al., 2010 [12], 2012 [13]). Rosenfeld et al. 2009 [14]) analyzed the chemical structure and isoforms of Hp isolated from Wadden Sea harbor seals, Grebe et al. analysed structure and isoforms of transferrin and obtained reference ranges for North Sea seals (Grebe et al. 2010 [15], 2011 [16], 2012 [17]).
Challenges
Anthropogenic influences may have an effect on the health status of animals. Reliable health parameters allow routine investigations of a large number of animals and provide information about these animals used as indicators of the coastal ecosystem, monitored in support of the Trilateral Monitoring and Assessment Program.
Related articles
- Common biomarkers for the assessment of marine pollution
- Endocrine system
- Elemental mass spectrometry - a tool for monitoring trace element contaminants in the marine environment
- Acoustic monitoring of marine mammals
- Coastal pollution and impacts
- Environmental risk assessment of marine activities
- Endocrine disrupting compounds in the coastal environment
- Portal:Ecotox
- Threats to Coral Reefs: the Effects of Chemical Pollution
References
- ↑ Jepson, P. D., Bennett, P. M., Allchin, C. R., Law, R. J., Kuiken, T., Baker, J. R., Rogan, E. & Kirkwood, J. K. (1999). Investigating potential associations between chronic exposure to polychlorinated biphenyls and infectious disease mortality in harbour porpoises from England and Wales. Science of the Total Environment, 244, 339-348.
- ↑ Bennett, P. M., Jepson, P. D., Law, R. J., Jones, B. R., Kuiken, T., Baker, J. R., Rogan, E. & Kirkwood, J. K. (2001). Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environmental Pollution, 112 (1), 33-40.
- ↑ Kakuschke, A. (2006). Einfluss von Metallen auf das Immunsystem von Meeressäugern. Dissertation, Universität Hamburg.
- ↑ Kakuschke, A., Valentine-Thon, E., Griesel, S., Fonfara, S., Siebert, U. & Prange, A. (2005). The immunological impact of metals in Harbor Seals (Phoca vitulina) of the North Sea. Environmental Science & Technology, 39 (19), 7568-7575.
- ↑ Kakuschke, A., Valentine-Thon, E., Fonfara, S., Griesel, S., Siebert, U. & Prange, A. (2006). Metal sensitivity of marine mammals: a case study of a gray seal. Marine Mammal Science, 22 (4), 985-997.
- ↑ Kakuschke, A., Valentine-Thon, E., Griesel, S., Fonfara, S., Siebert, U. & Prange, A. (2011). Are metal-induced hypersensitivities in harbor seals associated with liver function? Marine Pollution Bulletin, 62, 1891-1894.
- ↑ Kakuschke, A., Valentine-Thon, E., Fonfara, S., Griesel, S., Siebert, U. & Prange, A. (2008). Metal-Induced Impairment of the Cellular Immunity of Newborn Harbor Seals (Phoca Vitulina). Archives of Environmental Contamination and Toxicology,55 (1), 129-136. doi: 10.1007/s00244-007-9092-3
- ↑ Kakuschke, A., Valentine-Thon, E., Fonfara S., Kramer, K. & Prange, A. (2010). Influences of methyl-, phenyl-, ethylmercury and mercurychloride on immune functions in harbour seals. Journal of Environmental Sciences, 21, 1716–1721.
- ↑ Fonfara S., Siebert, U. Prange, A. & Colijn, F. (2007). The impact of stress on cytokine and Haptoglobin mRNA expression in blood samples from harbour porpoises (Phoconea phocoena). Journal of the Marine Biological Association of the United Kingdom, 87, 305-311.
- ↑ Fonfara S., Siebert, U., Prange, A (2007). Cytokines and acute phase proteins as markers for infection in harbour porpoises (Phoconea phocoena). Marine Mammal Science 23 (4): 931-942.
- ↑ Fonfara, S., Kakuschke, A., Rosenberger, T., Siebert, U. & Prange, A. (2008). Changes of cytokine and acute phase protein expression in blood samples of harbour seal pups during their first months of life. Marine Biology, 155, 337-345.
- ↑ Kakuschke, A., Erbsloeh, H.-B., Griesel, S. & Prange, A. (2010). Acute phase protein haptoglobin in blood plasma samples of harbour seals of the Wadden Sea and of the isle Helgoland. Comparative Biochemistry and Physiology, Part B, 155, 67–71.
- ↑ Kakuschke, A.; Pröfrock, D.; Prange, A. (2013). C-reactive protein in blood plasma and serum samples of harbour seals (Phoca vitulina). Marine Mammal Science, doi:10.1111/j.1748-7692.2012.00603.x
- ↑ Rosenfeld, H., Lassen, S. & Prange, A. (2009). Characterization of haptoglobin in the blood plasma of harbor seals (Phoca vitulina). Journal of Proteom Research, 8, 2923-2932.
- ↑ Grebe, M., Pröfrock, D., Kakuschke, A., Broekaert, J.A.C. & Prange, A. (2010). Metallomics approach for the identification of the iron transport protein transferrin in blood from harbor seals (Phoca vitulina). Metallomics, 2, 661-720.
- ↑ Grebe, M., Pröfrock, D., Kakuschke, A., Broekaert, J.A.C. & Prange, A. (2011). Fast and reliable absolute quantification of transferrin in blood samples of harbour seals using HPLC-ICP-MS. Metallomics, 3, 176-185.
- ↑ Grebe, M., Pröfrock, D., Kakuschke, A., del Castillo Busto, M.E., Montes-Bayon, M., Sanz-Medel, A., Broekaert, J.A.C. & Prange, A. (2012). Comparison of different methods for the absolute quantification of harbour seal transferrin glycoforms using HPLC-ICP-MS. Journal of Analytical Atomic Spectrometry, 27, 440-448.
Please note that others may also have edited the contents of this article.
|